Ohr Pharmaceutical has enrolled the first 60 patients in its Squalamine eye drop OHR-002 phase II trial to treat the wet form of age-related macular degeneration (wet-AMD).
Designed to assess the efficacy and safety of Squalamine eye drops, the randomised, double-blind study aims to enrol 120 treatment-naive wet-AMD patients at over twenty clinical sites in the US.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Ohr CEO Dr Irach Taraporewala said: "This milestone sets the stage for the planned interim analysis once the 60 patients complete the nine month treatment protocol and we expect the data to be available in the second quarter of 2014."
The Wet-AMD study will treat the patients with Squalamine eye drops or placebo eye drops two times a day for a period of nine months.
Visual acuity parameters, the need for rescue intravitreal injections and safety are the primary and secondary endpoints of the placebo-controlled study. The protocol of the study includes an interim analysis of the completion of the treatment period in 50% of patients.
Ohr scientific advisory board member and Vitreous-Retina-Macula Consultants of NY retinal disease specialist Dr Jason Slakter said: "The Company’s eye drop for treating wet-AMD would potentially offer patients a convenient, self-administered treatment alternative or adjunct to currently-used intravitreal injections directly into the eye."

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
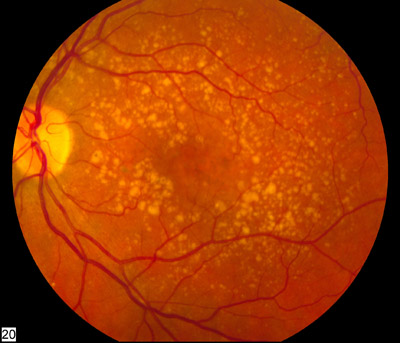
By GlobalDataImage: A fundus photo showing intermediate age-related macular degeneration. Photo: National Eye Institute of the NIH.





