Oxbryta™ (voxelotor), previously known as GBT440, is the first and only FDA-approved sickle haemoglobin polymerisation inhibitor indicated for the treatment of sickle cell disease (SCD).
Developed by Global Blood Therapeutics (GBT), the drug is the disease-modifying therapy for targeting the fundamental cause of SCD damage in adults and children of 12 years and older.
Oxbryta approvals
New drug application (NDA) filing was accepted by the US Food and Drug Administration (FDA) in September 2019. In November 2019, voxelotor received accelerated approval from the FDA under the subpart H pathway.
The approval came three months ahead of the priority review action date, ensuring the patients to access the drug. Priority review was also granted by the FDA for voxelotor with a review time of six months, while the Prescription Drug User Fee Act (PDUFA) target action date was set for 26 February 2020.
The drug received the Priority Medicines (PRIME) designation from the European Medicines Agency (EMA) for the treatment of SCD. It also obtained breakthrough therapy designation from the FDA.
Oxbryta is available as 500mg tablets via oral administration, once daily. The medicine made available through GBT’s speciality pharmacy partner network and patients in the US from December 2019.
Sickle cell disease (SCD) causes and symptoms
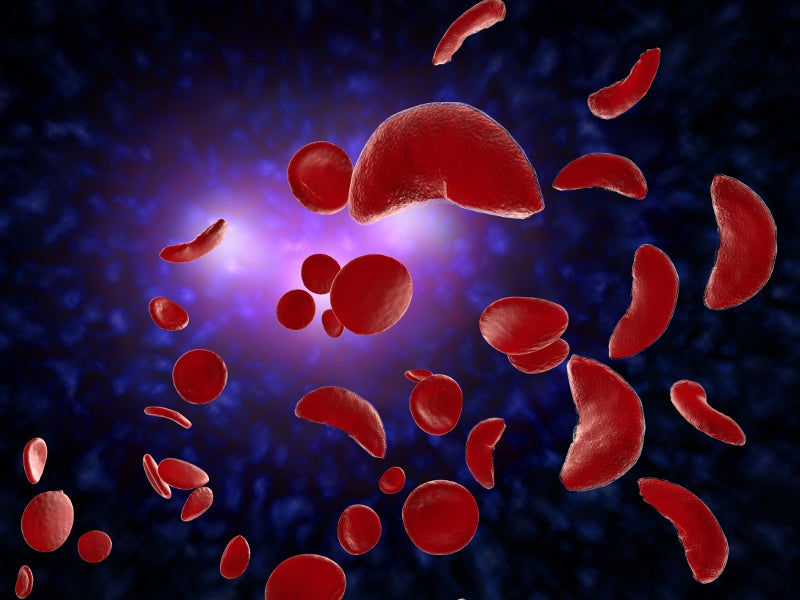
Sickle cell anaemia (SCD) is a group of inherited rare blood disorders that mainly affect red blood cells (RBCs) or erythrocytes.
RBCs of patients with SCD become deoxygenated, rigid and sticky. Furthermore, their shape is changed from round to crescent, or C-shaped farm tool referred to as “sickle”, resulting in a shortage of RBCs and leading to multiple organ failure and early death.
The most common types of SCD are HbSS, HbSC, HbS beta-thalassemia, HbSD, HbSE and HbSO.
The signs and symptoms of SCD include white (icterus) or yellow-coloured skin (jaundice), fatigue and painful swelling of hands and feet called dactylitis.
Voxelotor mechanism of action
Voxelotor is a polymerisation inhibitor of haemoglobin S (HbS) that modulates haemoglobin. It increases the oxygen affinity for Hb and thus exhibits dose-dependent HbS polymerisation inhibition.
Increased oxygen affinity stabilises RBCs in an oxygenated stage, preventing haemoglobin polymerisation to counter RBC destruction.
It also inhibits sickling of RBCs, improves deformability of RBCs and decreases whole blood viscosity.
Clinical studies on Voxelotor
Oxbryta (Voxelotor) approval from the FDA comes from positive results of the Phase 3 HOPE (Haemoglobin Oxygen Affinity Modulation to Inhibit HbS Polymerisation) clinical study.
Phase 3 HOPE (GBT440-031) is a randomised, double-blind, placebo-controlled, multicentre clinical trial involving 274 patients with sickle cell disease. A total of 90 patients received 1500mg of voxelotor, 92 patients received 900mg and the remaining 92 patients received a placebo during the study.
Effectiveness was based on the elevation in the haemoglobin response rate after 24 weeks of treatment of patients who received 1,500mg of voxelotor, with more than 51.1% of patients achieving an increased rate of 1g / dl, when compared to 6.5% in the placebo group.
The most common side-effects include nausea, diarrhoea, fatigue, headache, abdominal pain, rash and fever or pyrexia.
FDA also granted accelerated approval study for voxelotor for the treatment of SCD. As a result, GBT will continue to evaluate the drug in the HOPE-KIDS 2 Study, which is a post-approval confirmatory study using transcranial Doppler (TCD) flow velocity to reduce the risk of stroke in children aged between two and 15 years.
Marketing commentary on Global Blood Therapeutics (GBT)
Based in South San Francisco, GBT is a biopharmaceutical company involved in the exploration, development and manufacturing of innovative therapies.
The company currently has one FDA-approved therapy for the sickle cell disease (Oxbryta), and an investigational candidate (Inclacumab), a novel fully human monoclonal antibody for the treatment of vaso-occlusive crisis (VOC). The Inclacumab pivotal study is anticipated for 2021.
GBT also intends to expand complementary pipeline in the future.