US Flu Vaccine Efficacy: Cause for Concern
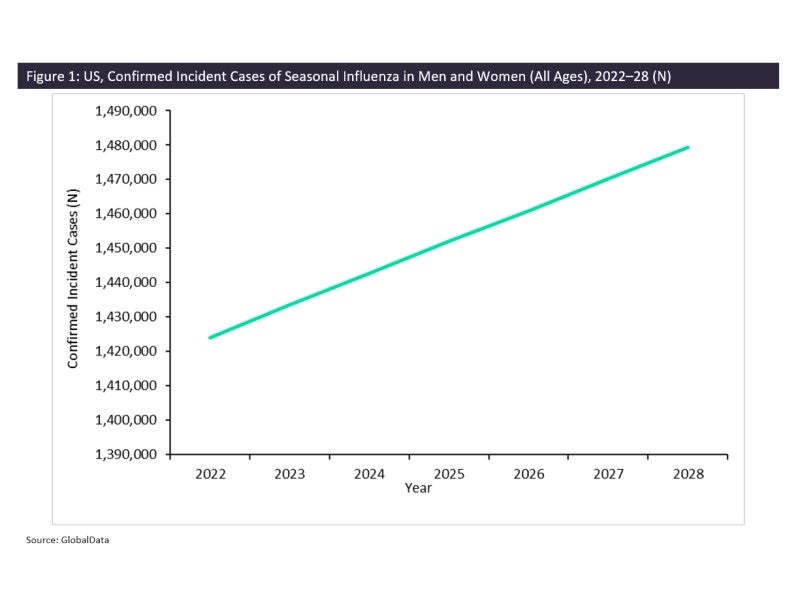
The currently available flu vaccines protect the population against the four most common influenza viruses in circulation: A(H1N1)pdm09, A(H3N2), B/Victoria lineage and B/Yamagata lineage. In the US, all flu vaccines are quadrivalent, and vaccination is recommended to all persons six months and older, unless advised otherwise by a medical professional. Influenza viruses infect the nose, throat, and occasionally the lungs and are responsible for causing seasonal flu epidemics. In the US, peak infection rates occur in fall and winter, and the virus spreads from person to person via droplets when an infected individual coughs or sneezes, and can live on surfaces for 24 hours. GlobalData epidemiologists estimate there will be 1.4 million incident cases of seasonal influenza in men and women in the US by the end of 2022; this number is forecast to increase to nearly 1.5 million by 2028 (as shown in Figure 1). According to a recent Centers for Disease Control and Prevention (CDC) report, the 2021-22 seasonal flu vaccine was not significantly effective in protecting the US population from contracting the most common influenza virus in current circulation.
Data on vaccine effectiveness (VE) was obtained from the March CDC Morbidity and Mortality Weekly Report. VE was calculated using data from the 3,363 children and adults with an acute respiratory infection (ARI) enrolled in the US Influenza Vaccine Effectiveness Network across seven different study sites in the US from October 2021 to February 2022. A VE of only 16% was observed from the 2021-22 seasonal flu vaccine in protecting the US population from contracting the most common influenza virus in current circulation, A(H3N2). More specifically, VE against mild to moderate ARI associated with the influenza A(H3N2) virus in outpatients, who received medical attention, was 16%, which was not significantly effective. Further, VE against outpatients with ARI associated with influenza A virus types, who were medically attended, was even less significantly effective at 14%. The low vaccine efficacy in the most common influenza strain in circulation is particularly concerning, as the A(H3N2) influenza virus mutates faster and typically leads to more hospitalisations and deaths, supporting the argument for continued influenza diagnostic testing, antiviral drugs, and preventative measures to mitigate its spread and resulting health complications.
Although the numbers may sound concerning, flu activity in the US this fall and winter has been considerably lower than what is traditionally seen. This can be largely attributed to the surge in Covid-19 Omicron cases in the winter of 2021 going into 2022, which caused people to wear face masks again and practice social distancing and hand washing – all preventative measures to take for reducing the spread of influenza viruses.
Despite this season’s low VE, the CDC continues to recommend flu vaccine uptake because growing evidence suggests serious outcomes and complications, including hospitalisation, ICU admission and possible death, in those who fall ill with influenza A(H3N2) virus infection, can be prevented with influenza vaccination. Furthermore, flu vaccination can reduce the incidence of infection by other influenza viruses that may come into mainstream circulation later on, such as A(H1N1)pdm09 and B viruses.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData