Abatacept (Orencia) was developed by Bristol-Myers-Squibb for the treatment of rheumatoid arthritis (RA). It is a biologic drug which contains a selective co-stimulation modulator.
The US Food and Drug Administration approved the drug in December 2005, followed by the European Commission in July 2010.
In August 2011, the FDA approved a subcutaneous (SC) formulation of abatacept for treating adults with moderate to severe RA.
Rheumatoid arthritis (RA)
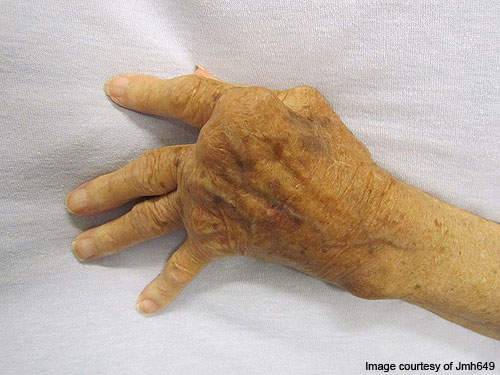
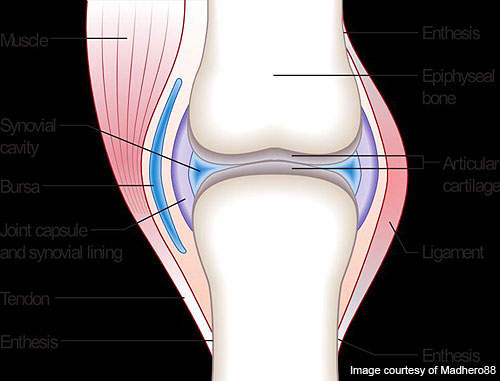
RA is a long-lasting, painful autoimmune disorder that can have an impact on organs and tissues within the body, especially synovial joints.
The progression of the disease leads to ankylosis and articular cartilage problems in the joints. The disease can also cause diffusing pains in the pericardium, nodular lesions, sclera, pleura and the lungs. It is a disabling and inflammatory condition which may lead to a loss of mobility.
The disease affects about one percent of the world’s population, with women three times more likely than men to have it.
About 75% of diagnosed RA patients are women. The disease can affect people of any age but more so in the 40-50 age group.
Targeting the disease with abatacept
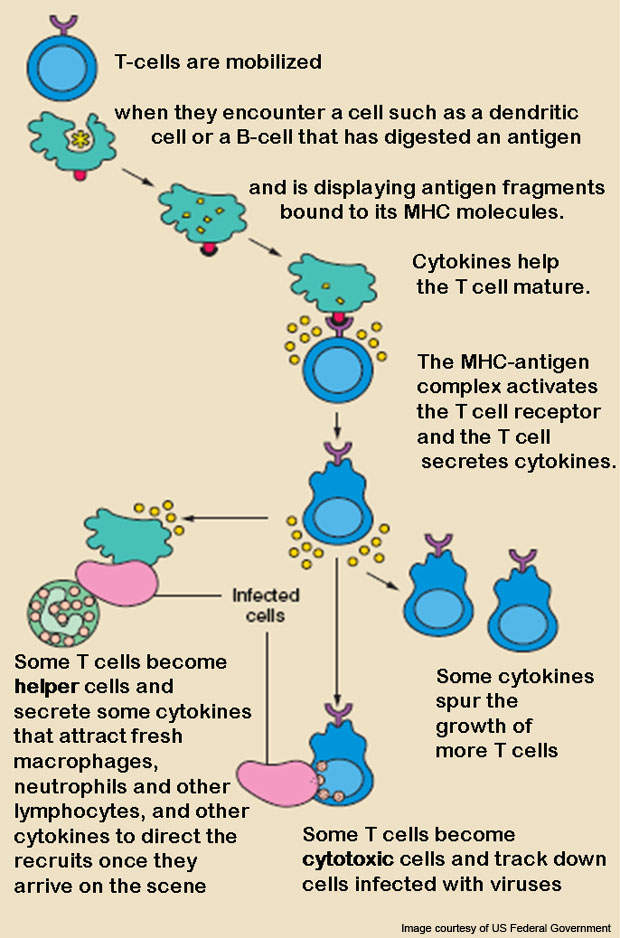
Abatacept is a prescription medicine developed to treat RA. The drug is a protein with immunoglobulin that contains a T-cell co-stimulation modulator. The activated T-cells play an organising role in the immunopathological mechanisms of RA.
Inhibiting full activation of T-cells is, therefore, necessary for treating RA. The drug works by selectively modulating a co-stimulatory signal that is necessary for T-cell activation. The drug is developed by applying recombinant DNA technology.
Clinical trials on Bristol-Myers Squibb’s orencia
Bristol-Myers Squibb began Phase I clinical trials in 2004-05. The objective of the trial was to study and estimate the tolerability and safety of the drug.
Twenty-five RA patients were enrolled for treatment with single and multiple doses of abatacept. The results of the study showed good tolerability in RA patients who were given single and multiple doses of the drug.
Phase II trials were conducted between January 2006 and May 2007. Sixty-eight RA patients were enrolled for the study and were given either a placebo or abatacept for the treatment. The results showed that 51 abatacept-treated subjects were seropositive for anti-abatacept antibodies.
The efficacy and safety of abatacept was studied in clinical trials by enrolling more than 2,600 RA patients. The trials included placebo-controlled and open-label extension periods.
Related project
Actonel (Risedronate Sodium) – Treatment for Osteoporosis, US
Actonel is targeted for the treatment of osteoporosis in postmenopausal women.
Phase III clinical trials started in April 2007 and concluded in July 2008. The trial included three major studies on AIM (abatacept in inadequate responders to methotrexate), ATTAIN (abatacept trial in treatment of anti-TNF inadequate responders) and ASSURE (abatacept study of safety in use with other RA therapies).
Phase III pivotal and efficacy studies of the drug showed a significant improvement in signs and symptoms of RA patients.
The American College of Rheumatology measured the results by using 20, 50 and 70 scores.
The results of the study indicated that the patients administered with abatacept showed an improved difference from placebos by day-15 for ACR 20 in some patients.
Related project
LODOTRA – Treatment for Rheumatoid Arthritis
LODOTRA was developed utilising SkyePharma’s proprietary GeoClock and GeoMatrix technologies, for which Horizon Pharma holds an exclusive worldwide license.
The Phase III study included a questionnaire which assessed how abatacept users improved in eight quality-of-life domains when compared with placebo patients. A significant portion of abatacept patients achieved an ACR 70 score for six months in comparison with methotrexate. Structural damage also decelerated in these patients.
Bristol-Myers Squibb conducted a head-to-head Phase IIIb clinical trial to compare abatacept and adalimumab (Humira). It is known as AMPLE (abatacept versus adalimumab comparison in biologic-naïve rheumatoid arthritis (RA) subjects with background methotrexate) study. It was a randomised, investigator-blinded multinational study.
It enrolled 646 adult biologic-naïve patients with active moderate to severe RA. The duration of the study was 24 months with a 12 month efficacy primary endpoint. The number of patients administered with abatacept plus MTX group was 318, while 328 patients were treated in the Humira plus MTX group.
The results of the study, announced in June 2012, showed that abatacept demonstrated better efficacy over humira (adalimumab) in treating moderate and severe RA patients.
Marketing commentary on rheumatoid arthritis treatment drugs
Sales of abatacept have increased steadily in the US since 2006. Moreover, in June 2010, Pfizer decided to stop the development of one of the two investigational drugs that it is manufacturing with Trubion Pharmaceuticals for treating RA.