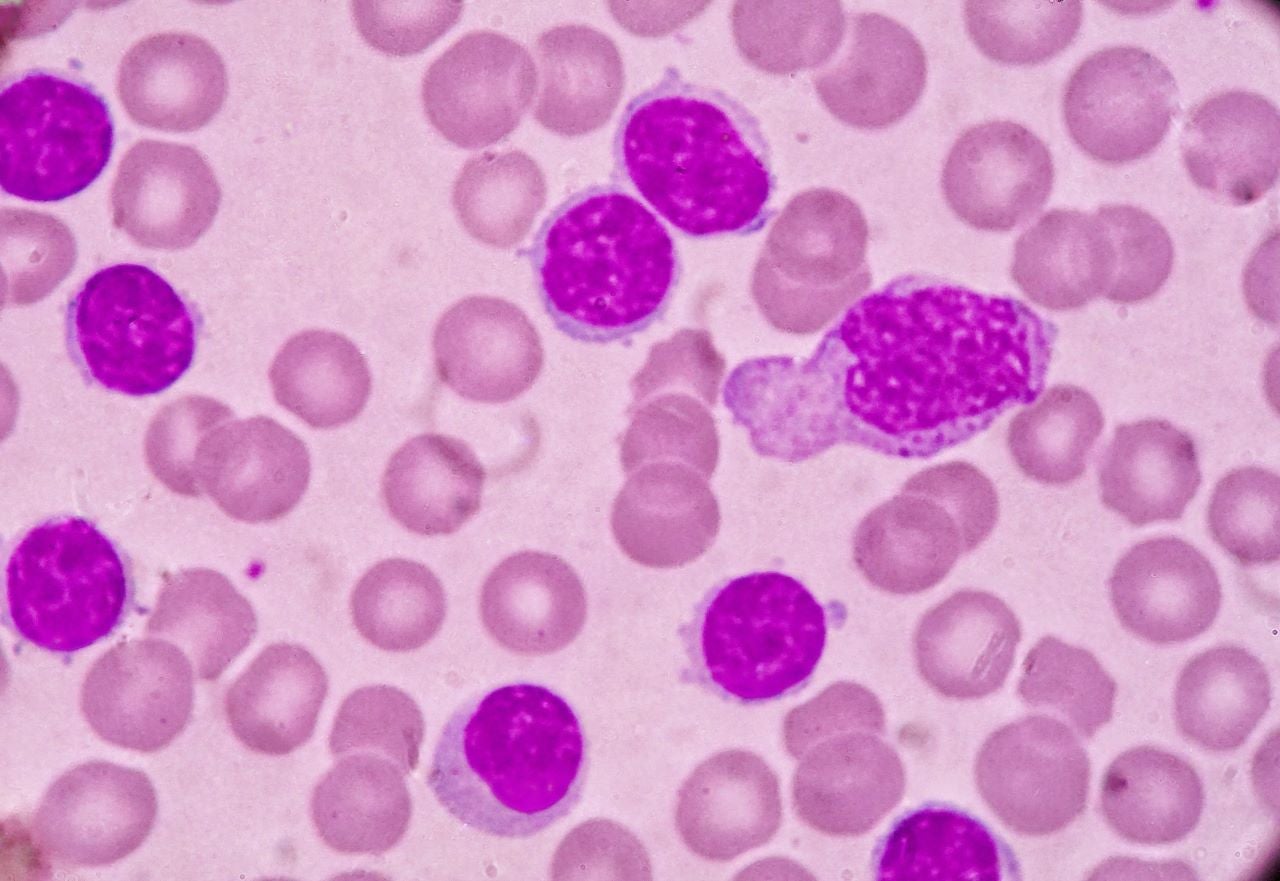
At the American Society of Clinical Oncology (ASCO) Annual Meeting on 2-6 June 2023, results were presented from the Phase I/II TRANSCEND CLL 004 trial investigating Breynazi (lisocabtagene maraleucel) for the treatment of relapsed/refractory (R/R) chronic lymphocytic leukaemia (CLL) or small lymphocytic lymphoma (SLL). CLL and SLL both arise from abnormal lymphocytes in the bone marrow. In CLL the malignant cells are found in the blood and bone marrow, while in SLL they reside in the lymph nodes. CLL and SLL are among the most common haematological malignancies in adults, with an estimated 18,740 new cases expected in 2023 in the US alone. Significant progress has been made in the management of CLL/SLL with the use of Bruton tyrosine kinase (BTK) inhibitors, B cell lymphoma 2 (BCL2) inhibitors, and phosphatidylinositol 3-kinase (PI3K) inhibitors; however, these therapies are not considered to be curative. Once patients become refractory or intolerant of BTK and BCL2 inhibitors, there is no established standard of care, with patients having a poor prognosis. This creates a high level of unmet need in the R/R CLL/SLL setting.
The TRANSCEND CLL 004 trial enrolled 137 patients with R/R CLL/SLL who underwent leukapheresis. Of these, 117 were treated with Breyanzi, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy. All 117 patients were evaluable for safety, and 96 were evaluable for efficacy. The median age of the study participants was 65, with 44% of the patients having bulky lymph nodes, and 83% having high-risk cytogenetics. Patients had received a median of five prior lines of therapy, with 88% refractory to a BTK inhibitor; 76% refractory to AbbVie and Genentech’s Venclexta (venetoclax), a BCL2 inhibitor; and 60% experiencing progression on a BTK inhibitor and experiencing Venclexta failure.
The independent review committee (IRC)-assessed complete response (CR) rate among all patients, including those treated at dose level one, was 18%, and the IRC-assessed overall response rate (ORR) was 48%. The undetectable minimal residual disease (MRD) rate in blood was 62%, and the undetectable MRD in marrow was 59%. The median time to first response was two months, and the median duration of response was 35.25 months. The median duration of CR or remission was not reached. The median overall survival (OS) after 36 events had occurred during the trial was 43.17 months. In the BTK inhibitor progression and venetoclax failure subset (n=53) the results were similar, with a CR rate of 19%, an ORR of 43%, an undetectable MRD in blood rate of 62%, and an undetectable MRD in marrow of 59%. The median duration of response was also 35.25 months for this subset, and the median duration of CR or remission was not reached. The median OS was shorter for this subset, at 30.26 months, but at the time of data cut-off, only 22 events had occurred. Regarding safety data, 92% of patients experienced a grade ≥3 treatment-emergent adverse event (TEAE), with the most common being neutropenia (61%), anaemia (52%), and thrombocytopenia (41%). Of the five deaths that were due to TEAEs, only one death was considered related to the Breyanzi by the investigator. Importantly, only 9% of patients had grade 3 cytokine release syndrome and no grade 4 or 5 cytokine release syndrome occurred. Grade 3 neurological events occurred in 18% of patients, and only 1% had a grade 4 neurological event.
This data demonstrates Breyanzi to be a strong therapeutic candidate for patients with R/R CLL/SLL who have progressed on BTK and BCL2 inhibitor therapy, and it is anticipated that Breyanzi will become the first CAR-T cell therapy to gain approval in this setting. Approval would provide a potentially curative therapy to patients who have limited treatment options and would also be a significant win for BMS, with GlobalData’s patient-based forecast predicting that Breyanzi sales in the CLL/SLL setting will reach $309m in the eight major markets (8MM: US, France, Germany, Italy, Spain, UK, Japan, and China) by 2031.
See Also:
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData






Related Company Profiles
AbbVie Inc