Patient centricity refers to the process of designing a clinical trial, product, or service around a patient’s needs. Recently, there has been a surge towards recognising the correlation between patient experience and trial success. This has resulted
in patient-centric drug development becoming the model that leaders in the industry are following.
According to research published by The Economist Intelligence Unit, titled The Innovation Imperative: The Future of Drug Development, drugs developed using patient-centric designs were more likely to be launched compared to drugs developed without. It was also reported that patient-centric trials take less time to recruit patients. This indicates that optimsing patient experience leads to faster recruitment and improved retention.
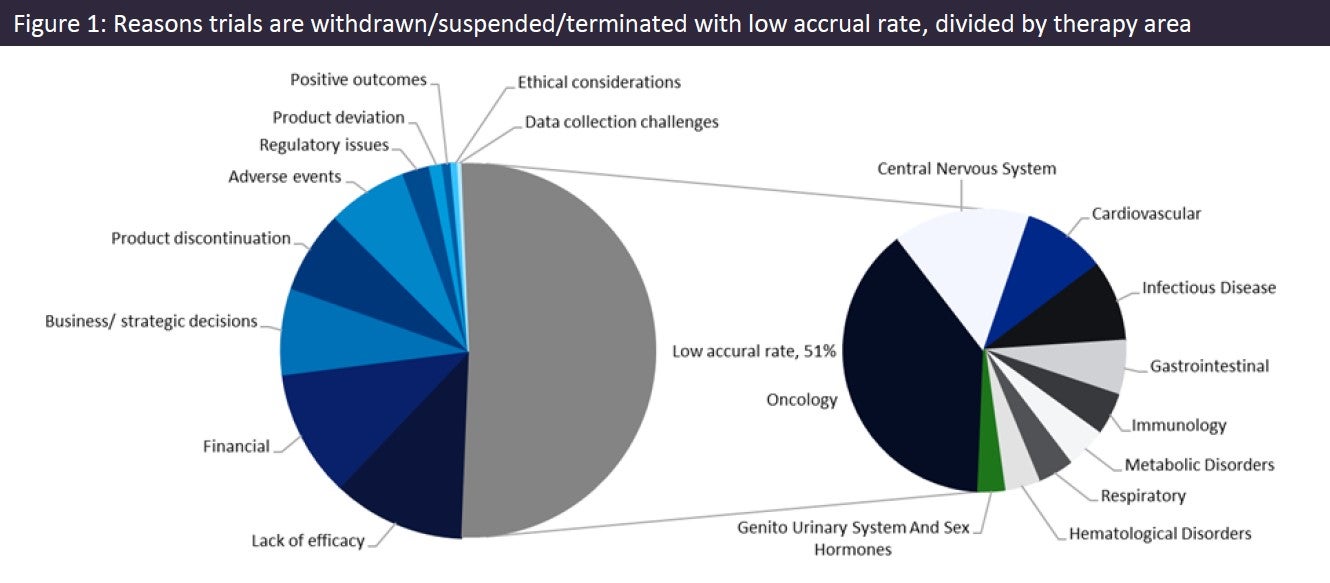
According to figures obtained from GlobalData’s enrolment module, only 46% of clinical trials have reported meeting their enrolment target. Data also revealed that insufficient accrual is the leading cause of trials being withdrawn/suspended/terminated and that oncology is the leading therapy area with the most accrual-related trial failures (Figure 1).
Clinical studies that involve patients commuting vast distances between trial sites and their homes or that require a high volume of visits increase the patient burden, which has been found to reduce trial recruitment and retention. The Covid-19 pandemic inadvertently demonstrated the legitimacy of decentralised clinical trials (DCT) to trial sponsors, which has manifested in the increased utilisation of virtual trials. Digital technologies such as telemedicine, remote patient monitoring, and wearable devices reduce patient burden. This may be why only 2% of DCTs have been withdrawn/suspended/terminated due to low accrual rates, according to GlobalData’s clinical trials database.
Other factors that hinder patient recruitment and retention in traditional trial models include a lack of patient support for explaining complex protocols and little or no reimbursement of expenses. These issues are being taken into consideration by trial sponsors who realise that trials with low accrual rates are frequently unable to inform clinical practice or benefit patients. This leads to a waste of critical resources, which ultimately increases costs for sponsors.










Related Company Profiles
The Economist Intelligence Unit Limited