Gastric cancer is an area of extreme unmet need where immuno-oncology (IO) treatments could provide a better survival outcome to patients. GlobalData has identified that Merck & Co and two company partnerships, Bristol-Myers Squibb (BMS)/Ono and Merck KGaA/EMD Serono/Pfizer, are leading the development of immune checkpoint modulators in gastric cancer.
Among the 14 Phase II/III and III trials in gastric cancer assessing immune checkpoint modulators, eight are part of currently ongoing trials exploring the potential of immune checkpoint inhibitors in combination with either standard of care chemotherapy or other IO agents. GlobalData expects this strategy to be practice-changing for gastric cancer patients over the next three years.
Number of company-sponsored phase II/III and III clinical trials in gastric cancer exploring immune checkpoint modulators, by sponsor
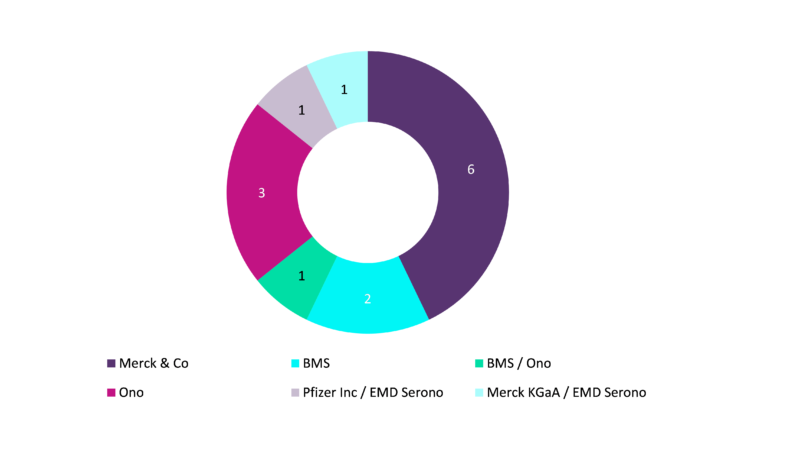
Source: GlobalData. Note: The number of late-stage clinical trials in gastric cancer includes all global Phase II/III and III trials sponsored by companies.
In September 2017, Merck’s Keytruda (pembrolizumab) and BMS/Ono’s Opdivo (nivolumab) were both approved in heavily pretreated patients—the third line treatment of metastatic disease—by the FDA and Japan’s Ministry of Health, Labour and Welfare (MHLW), respectively.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataWhile all trial sponsors are trying to establish immune checkpoint modulators as new standard of care treatments in earlier settings, and therefore associating them with a larger number of gastric cancer patients, significant challenges remain to demonstrate survival benefit in the first or second lines of therapy.
In December 2017, two pivotal Phase III trials exploring immune checkpoint modulators in monotherapy failed to show any survival benefit, including Keytruda in the second line in the KEYNOTE-061 trial and Merck KGaA/Pfizer’s Bavencio (avelumab) in the third line of the metastatic setting in the JAVELIN Gastric 300 trial.
The competitive landscape in gastric cancer is rapidly changing, and GlobalData forecasts immune checkpoint modulators to show significant clinical and commercial impact by 2020.




