Takeda and its partner, Zinfandel, have announced that the investigation of Actos (pioglitazone) in the global Phase III TOMMORROW trial, one of the largest studies ever to be executed in mild cognitive impairment (MCI) and Alzheimer’s disease (AD), has been terminated.
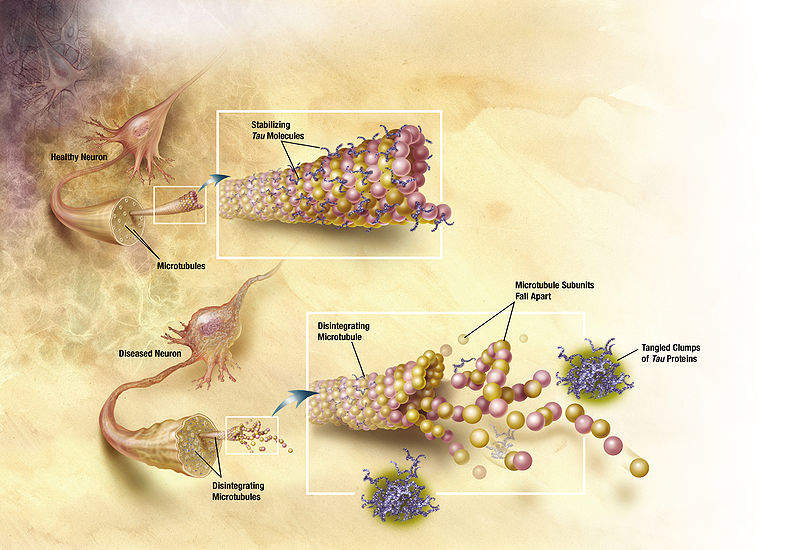
AD represents the most common neurodegenerative dementia in the elderly population and is a progressive neurodegenerative disease. During the development of AD, the degeneration of neurons starts in the brainstem before progressing to the hippocampus, frontal lobe, amygdala, parietal lobe, and temporal lobe. The cause of AD is not known, but appears to be multifactorial, with most research aimed at the amyloid beta (Aβ) peptides and tau proteins. Disease onset is usually characterised by memory loss for recent events, and is often associated with repetitive questioning and loss of ability to learn new concepts.
Several failures in AD clinical trials have encouraged researchers to look at treatment in the pre-symptomatic phase, and pioglitazone is an anti-inflammatory drug that inhibits the depositing of harmful proteins in the brain, which gives it a preventive effect on the development of AD.
The TOMORROW trial
The TOMMORROW Phase III clinical trial was a global, multi-centre, randomised, double-blind, placebo-controlled, parallel-group trial in individuals between the ages of 65 and 83. The study enrolled approximately 3,500 people across 50 centres in the US, UK, Germany, Switzerland and Australia, who were then randomised into the trial.
The study had two separate goals. The first was to evaluate how accurately the biomarker risk assignment algorithm (BRAA)ased on the ApoE and TOMM40 genes, predicts a person’s risk of developing MCI due to AD within five years. The second was to evaluate the efficacy of the investigational agent, pioglitazone 0.8mg SR, in delaying the onset of MCI due to AD in projected high-risk individuals, as determined by the BRAA.
According to Takeda, the decision to end the trial was not related to the safety of the investigational product or study procedures, but was instead due to an inadequate treatment effect with the investigational drug, pioglitazone 0.8 mg SR, in delaying the onset of MCI due to AD.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataTakeda’s head of neuroscience Emiliangelo Ratti stated: “Takeda and Zinfandel will further analyze data from the trial, including the performance of the genetic-based biomarker risk assignment algorithm, with the hope that this information may ultimately help in the global fight against AD. Takeda remains committed to the discovery and development of potential treatments for AD.”
Takeda’s future in AD research
This commitment is demonstrated by the fact that Takeda has another drug under development for AD, TAK 071, which is in Phase I, as well as by the latest partnership between Takeda and Denali Therapeutics. This partnership is intended to develop treatments for AD and other neurodegenerative diseases, and to incorporate Denali’s Antibody Transport Vehicle (ATV) platform for increased exposure of biotherapeutic products in the brain into Takeda’s portfolio.
Despite the fact that key opinion leaders (KOLs) have highlighted pioglitazone has a potential as a disease-modifying therapy (DMT), its previous failure in a Phase II trial has reduced excitement around the drug. According to a KOL interviewed by GlobalData, “[Pioglitazone] was a drug that, basically, failed a large Phase II trial, and from a post-analysis of that trial, one of the doses was carried forward.”
Another interviewed KOL also voiced concerns around the trial design of the TOMORROW trial, which changed the sample size drastically in the middle of the study, saying: “They jumped in with a huge multi-thousand patient prevention trial without having a huge amount of data to support it.“
GlobalData believes that even though pioglitazone had potential as a DMT, it lacks the specificity to be useful in the AD space.
Related Reports
GlobalData (2017). PharmaPoint: Alzheimer’s Disease – Global Drug Forecast and Market Analysis to 2026, August 2017, GDHC149PIDR