EvidentIQ is a next-generation e-clinical technology platform that provides direct access to patients via our own patient community, which covers more than 500,000 patients with 1,200 chronic diseases in the EU and US.
Our e-clinical platform has been developed in a patient-centric manner that combines advanced technology with patient data, allowing us to support our customers in traditional, hybrid, and decentralised trials.
EvidentIQ is one of the few companies that offer a comprehensive, integrated approach to clinical development. Our capabilities include electronic data capture (EDC), randomisation and trial supply management (RTSM), clinical trial management systems (CTMS), electronic trial master files (eTMFs), electronic clinical outcome assessments (eCOAs), e-consent, electronic patient-reported outcomes (ePROs), and medical imaging.
End-to-end clinical solutions designed for ease of use
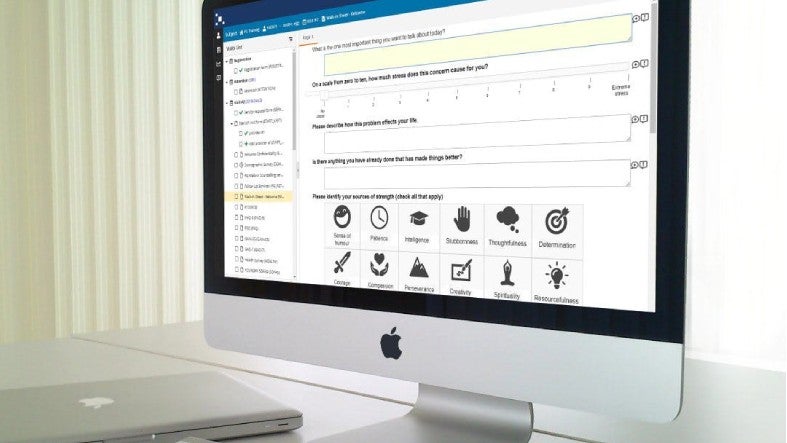
EvidentIQ’s eClinical Suite provides various features for the control and monitoring of captured data through real-time dashboards, integrated business intelligence (BI) capabilities, and automated report generation.
In the clinical planning phase, we can provide protocol writing or optimisation services and feasibility studies to answer questions such as ‘Can I find the required number and diversity of patients in a certain country?’ or ‘Will my inclusion/exclusion criteria allow me to find enough patients?’. By taking this approach, we help our clients optimise their protocols and reduce costs.
For conducting clinical studies, we offer a seamless e-enrolment workflow that involves identifying patients in our community, pre-screening them, and collecting their consent via our e-consent application. Our e-consent programme contains video conferencing capabilities to enable an informed consent discussion between physician and patient.
Patients are subsequently set up automatically in our EDC, where they are randomised. Data collection can then start via our next-generation eCOA/ePRO/eDiary application, site or remote visits, or wearable devices.
We have conducted more than 3,500 studies with more than 2.5 million patients to date. Our customers are pharma/biotech and medical device sponsors, as well as cosmetics and nutrition companies, contract research organisations (CROs), and academic institutions.
Accurate, real-world patient data for optimised clinical outcomes
EvidentIQ is an expert in patient-reported outcomes and offers direct access to patients for all online and self-reported studies. We have a group of data science experts and a technological platform that allows us to give our clients the best-quality service and data.
Our expert data analysts bring an innovative way to digitally recruit patients for non-interventional studies and analyse their data, allowing clients to control their studies and generate the data they need.
EvidentIQ offers Carenity, a social platform that supports more than 500,000 patients and caregivers worldwide across more than 1,200 chronic and rare diseases. Carenity provides direct, timely and compliant access to patient real-world data for real-world evidence, clinical trial optimisation, and scientific communication and digital publications.
As a digital CRO, our mission is to engage patients online to uncover real-world evidence and advance medical research. We are convinced that patient insights bring value at each phase of the drug life cycle, and we have the tools to access those in the easiest and fastest way.
Decentralised trial services focused on patient centricity and digital innovation
EvidentIQ is aware of the increasing need for decentralised clinical trials (DCTs) in clinical research, both in full and hybrid forms. We combine unique technologies to offer an innovative DCT experience and optimise clinical research, expediting trials and improving efficiency while leveraging a safe, scalable, and secure infrastructure.
Our goal is to expand research to everyone through flexible technology. We enable direct access to patients through Patient Poll and our patient platforms, providing the tools needed to conduct a totally digital or hybrid trial through our eCOA, eConsent, and data science expertise.
Our solutions can also help clients with their DCT strategy definition, study design, patient recruitment, study monitoring, and biostatistics.