esqLABS
Software, Consulting and Workshops for Data Analysis and Model-Based Decision Support
Founded in 2017, esqLABS provides platform technologies, software, consulting and workshops for data analysis and model-based decision support throughout the healthcare industry.
Subscribed
You have successfully submitted your enquiry. Someone from our company will respond ASAP
About Us

Founded in 2017, esqLABS provides platform technologies, software, consulting and workshops for data analysis and model-based decision support throughout the healthcare industry.
The company focuses on research and development (R&D) consultancy services and solutions using physiologically based (PBPK) and quantitative systems pharmacology (QSP) modelling and simulation.
EsqLABS leverages open-source computational tools for the analysis of in-vitro, animal in-vivo and human in-vivo data to provide translational insights to R&D teams on the mode-of-action of the clinical effect of drug therapies and the toxicity of chemicals.
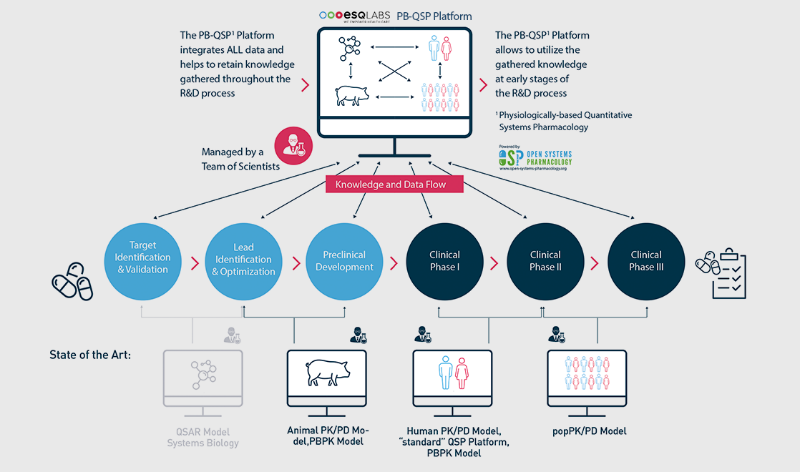
Quantitative knowledge integration through machine learning and computational platforms
esqLABS leverages machine learning and knowledge-based computational systems-biology and systems-pharma / toxicology platforms to predict tissue-specific exposure, efficacy and safety of chemicals and biologics at the site of action.
Our platforms aim to quantitatively integrate knowledge about compounds to gain an understanding of mechanisms of action in the context of environmental and clinical exposure-response relationships. These are leveraged throughout the development process of a chemical or drug.
We help translate multi-scale data from all sources and phases of drug development and deployment (omics, in-vitro and preclinical). This enables us to identify the relationship between drug exposure, safety / toxicity and efficacy parameters to determine a drug’s therapeutic window, establishing clinical dose regimens.
Furthermore, we enable proof-of-concept analyses, such as fast prototyping, and facilitate planning and optimisation of clinical development.
Open Systems Pharmacology (OSP) Suite software
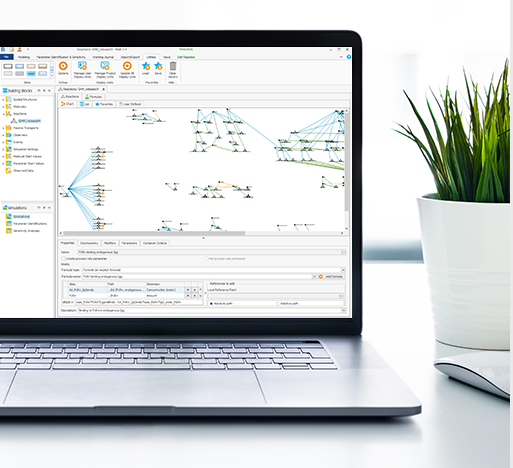
Our platform technology, built on top of the open-source software framework OSP suite, is jointly developed with other stakeholders from the pharmaceutical industry and academia.
This open-source framework enables peer-reviewed quality control through its community and complies with regulatory requirements through its next-generation platform qualification framework.
Our current customers are R&D teams within the pharmaceutical, chemical and crop protection industries as well as health technology Start-Ups.
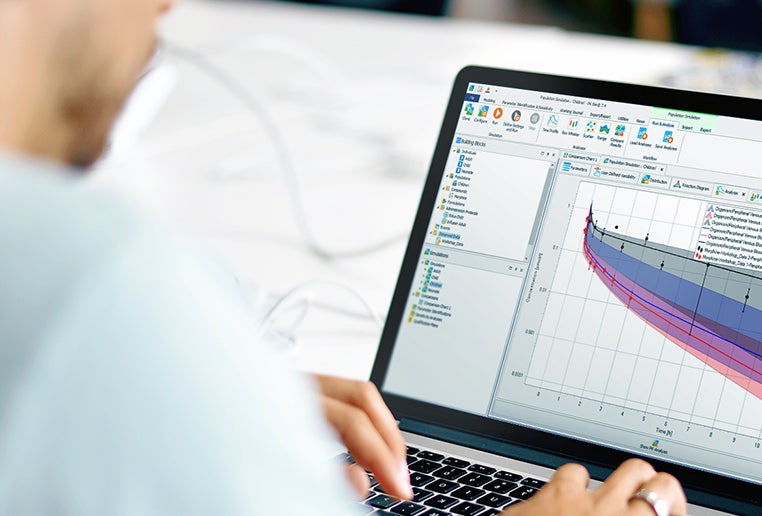
PBPK modelling and simulation services and training
EsqLABS provides services and consulting for standard applications of PBPK models using the OSP Suite with PK-Sim® and MoBi®.
Services include:
- Preclinical / clinical base PBPK model development
- Special populations: Organ impairment studies and PBPK-Based paediatric investigations
- Drug-drug interactions (DDI) investigations
- Translational modelling for first-in-man dose predictions
EsqLABS provides bespoke, individual training for customers on PBPK and PB-QSP modelling and simulation with PK-Sim® and MoBi®.
Platform technologies: QSP disease platforms
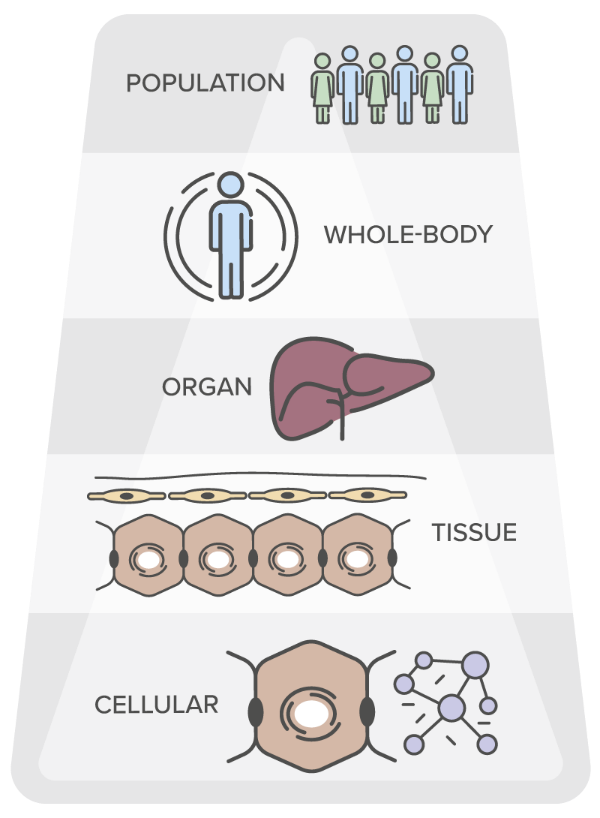
Trial simulations and in silico studies currently complement experimental studies in pharmaceutical R&D. Our software tools and project workflows typically integrate features of drug action such as pharmacodynamics interaction or pharmacokinetics at the organism and cellular scale, as biology always is multi-scale by nature.
The multi-scale physiologically-based (PB) modelling concept in our PB-QSP platforms and PBPK models enable the integration of disease, drug and physiological knowledge across various treatments within a therapeutic field, leveraging multi-scale data from all biological scales.
Through simulation and modelling, the multi-scale PB framework is leveraged in pharmaceutical, chemical, crop and healthcare technology industries for informed decision-making across all stages of R&D on the basis of a quality-controlled, consistent build-up and transfer of actionable knowledge.
Contact Details
Website
Email Address
Address
26683 Saterland,
Germany