Iomab-B for the treatment of Refractory and Relapsed Acute Myeloid Leukaemia
Iomab-B is a radio-immunotherapeutic drug developed by Actinium Pharmaceuticals for treatment of refractory and relapsed acute myeloid leukaemia in elderly patients.
BIOCON provides expert preclinical animal services to the biomedical research community. Our over 30 years of experience in providing quality housing, breeding, and technical manipulations of research animals is unsurpassed.
You have successfully submitted your enquiry. Someone from our company will respond ASAP
BIOCON provides expert preclinical animal services to the biomedical research community. Our over 30 years of experience in providing quality housing, breeding, and technical manipulations of research animals is unsurpassed.
We perform product safety evaluations, long and short-term carcinogen studies, as well as the production and manipulation of monoclonal antibodies, in vitro and in vivo.
We specialize in animal holding and breeding, immunology studies, acute and subchronic toxicity testing, product safety testing, contract management services for animal facilities, and custom antibody production.
Our animal breeding experience includes the production of over 40 congenic mouse strains and over 100 transgenic mouse constructs for the NIH, NCI, and commercial clients.
BIOCON provides assistance to its clients in the selection of appropriate animal models to meet their research needs.
Preclinical proof-of-concept studies often have unique technical requirements, and they can vary considerably in terms of their time and cost constraints. To accommodate these differences BIOCON typically designs custom protocols for its clients.
We follow a disciplined, structured methodology that solicits valuable client input at each stage. We also draw on our extensive, first-hand knowledge of small animal test species — all varieties of mice and rats, rabbits, guinea pigs, hamsters and ferrets — to present our clients with both traditional and innovative design formats. Our protocols are simple, elegant designs that make highly effective use of our clients’ resources.
Our general services include:
Our proof-of-concept testing services include:
BIOCON began working in the highly regulated government sector for such clients as the FDA, USDA, US military, CDC, and ten of the NIH institutes. Many of our government and commercial clients’ projects are now in clinical trials. We have an impeccable record of regulatory compliance, substantial in-house expertise and a quality-conscious program. Highlights include:
Regulatory compliance:
In-house capabilities:
Vaccine expertise:
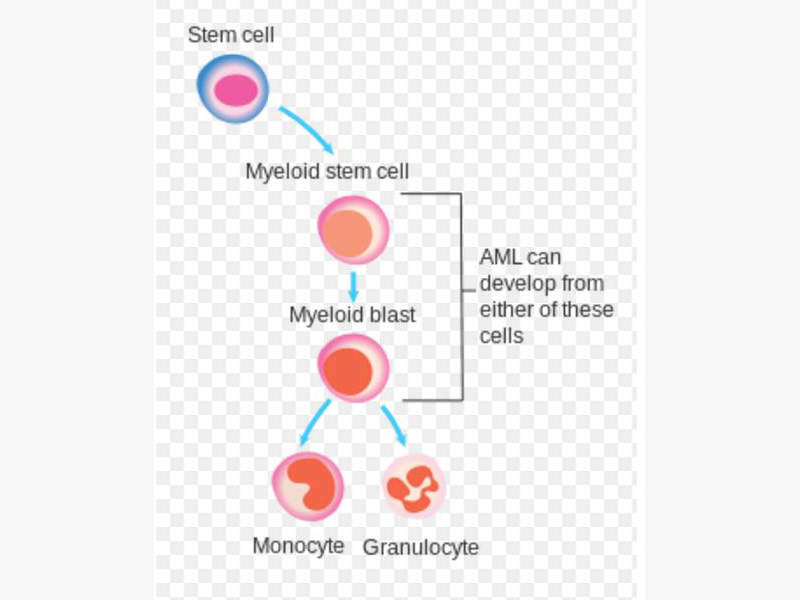
Iomab-B is a radio-immunotherapeutic drug developed by Actinium Pharmaceuticals for treatment of refractory and relapsed acute myeloid leukaemia in elderly patients.