Lumis International
Legal and Regulatory Consultation for Clinical Trials
Lumis International helps companies to expedite their clinical research activities in the European Union.
Subscribed
You have successfully submitted your enquiry. Someone from our company will respond ASAP
About Us

Lumis International was founded in 2013 with the goal of acting as a bridge to Europe for clinical research activities. Specialising in legal representation and regulatory consulting for life science companies outside Europe, we support companies in advancing their clinical development projects in the European market.
At Lumis, we provide legal and data representation for clinical trials in the EU, Switzerland and the UK, as well as legal representation during market authorisation to access the EU market, for companies registered as small to medium-sized enterprises (SMEs) with the European Medicines Agency (EMA).
We also offer support in obtaining and maintaining orphan drug designation, regulatory and operational consulting for entering the European clinical research market, and regulatory consulting expertise under the new EU Clinical Trial Regulation (EU-CTR) and Medical Device Regulation (MDR) specifications.
Up-to-date regulatory guidance for operations in the EU
Accessing the European biopharmaceutical and medical device markets can be both challenging and costly. Failure to comply with EU regulations can mean your product is denied access to the lucrative European market – an expensive waste of any company’s time. Furthermore, the EU’s 2022 clinical trial regulations can present challenges for sponsors to manage.
At Lumis International, we understand all this. Since 2013, we have been helping global biopharmaceutical and medical device companies access European markets more quickly, efficiently and cost-effectively.
With offices in Switzerland and the UK, we have a wide network of experts with in-depth knowledge of the EU’s regulatory landscape. Our UK office also supports clients in UK data representation.
Work with Lumis International to achieve a shorter time to market for innovative products, and cost savings when applying for SME status from the EMA. We utilise our in-depth knowledge and expertise in partnership by working across the whole clinical trial team.
Full compliance with EU medical device regulations
Regulation (EU) 2017/745 on medical devices (MDR) came into place on 26 May 2021. The EU has revised the laws governing medical devices to align with developments in the sector over the last 20 years. Its priority was to ensure a robust, transparent and sustainable regulatory framework, maintaining a high level of safety while supporting innovation.
With the EU MDR now in force, new devices will have to meet its requirements if manufacturers want to sell them on the European market.
Changes made under the MDR include an expansion of the products covered, more rigorous requirements for clinical evaluation, including changes to clinical investigations, mandatory unique device identification (UDI) mechanisms, and increased post-market oversight by EU Notified Bodies.
The already complex development process for medical devices, combined with these changes, is likely to make the transition complicated and time-consuming for most device manufacturers. Lumis International’s team of experts provides regulatory consulting services to support device manufacturers in this.
We can help with the early implementation of a strategic approach to ensure MDR compliance and make products more attractive to value-conscious buyers, handling technical documentation and implementing the required quality management system for the European market.
Conformity with regulations for clinical trials in Europe
To create a favourable environment for conducting clinical trials in the EU, with the highest safety standards for participants and increased transparency of trial information, the Clinical Trials Regulation (Regulation (EU) No 536/2014) (EU-CTR) will come into force on 31 January 2022. This will replace the EU Clinical Trials Directive (EC) No 2001/20/EC.
The EU-CTR promises a harmonised, simplified process designed to decrease the burden resulting from idiosyncratic interpretations of current regulations. It does, however, present significant operational challenges for sponsors. For example, sponsors must establish processes for document redaction. If companies prepare well in advance, they will find that they can initiate and conduct clinical trials in a timely fashion under the EU-CTR.
Lumis International is ready for this update and will provide continuous support for clients to ensure their clinical trial projects comply with the EU-CTR.
Consultation services for life science activities
Lumis Life Science Consulting was founded in 2020 as a subsidiary of Lumis International. The company focuses on providing consulting services for medical device and biopharmaceutical SMEs, as well as larger biopharmaceutical companies, when outsourcing their clinical activities.
Our services cover the whole spectrum of vendor selection and management, clinical trial strategy, clinical and quality oversight, sponsor-vendor conflict resolution, translation from preclinical to clinical development, regulatory consulting, and training.
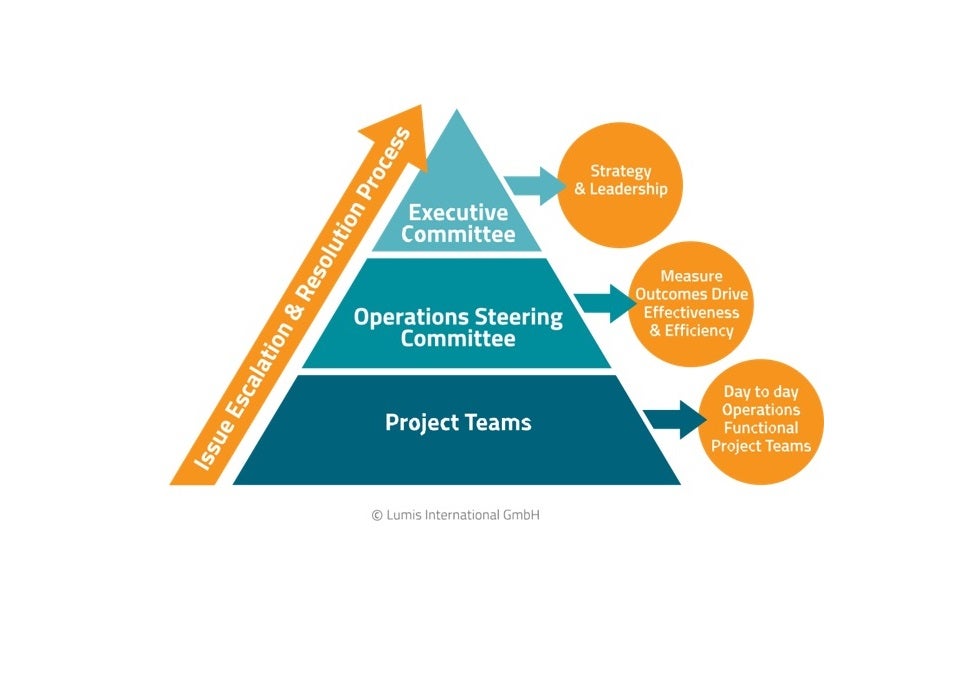
Outsourcing a clinical research project can be complex and fraught with issues. From addressing regulatory demands to monitoring your key performance indicators (KPIs) and monitoring vendor performance, the learning curve is steep and the challenges are consistent. That is why our solutions are tailored around you and designed to expedite your development project at every stage.
The senior team at Lumis Life Science Consulting has a proven track record in the pharmaceutical, biopharmaceutical and medical device sectors. In addition, we have a network of experienced consultants to collaborate with on niche tasks.
Faster and more cost-effective clinical trial management
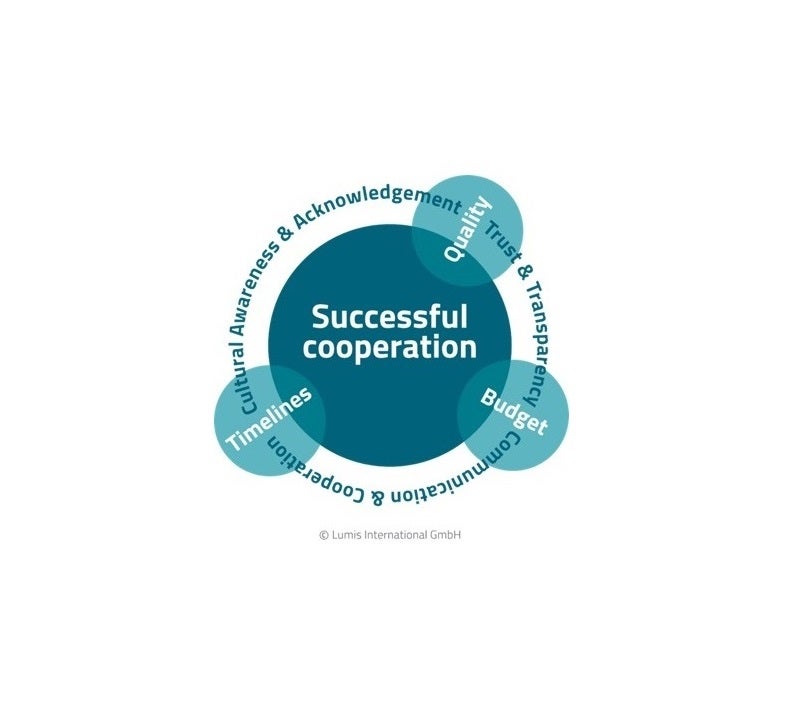
Too many clinical trials and development projects collapse due to a mismatch in priorities and understanding between the parties involved. Lumis International and Lumis Life Science Consulting are experts in navigating these challenges to manage expectations and align objectives, facilitate communication, and mitigate confrontation.
Work with Lumis Life Science Consulting to achieve a shorter time to market for innovative products, a realistic budget without compromising on quality, and a harmonious sponsor-vendor team all striving for the same goal.
Flexibility and empathy are core to the Lumis brand. We aim to go the extra mile in supporting our clients.
Contact Details
Website
Email Address
Address
10629 Berlin,
Germany