TG Therapeutics began Phase II trial of TGR-1202 and ibrutinib combination to treat DLBCL
US-based biopharmaceutical company TG Therapeutics began its Phase II investigator initiated trial of TGR-1202 in combination with ibrutinib to treat relapsed or refractory Diffuse Large B-cell Lymphoma (DLBCL).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
TGR-1202 is an orally administered PI3K delta inhibitor programmed to target the delta isoform with nanomolar potency and selectivity over the alpha, beta, and gamma isoforms of PI3K.
The delta isoform of PI3K is expressed in cells of hematopoietic origin and considered responsible for progression and survival of B-cell lymphocytes.
Study found antidepressant use increases hip fracture risk among patients with Alzheimer’s disease
The findings of a study conducted by the University of Eastern Finland suggested that antidepressant usage increases the risk of hip fracture in patients with Alzheimer’s disease residing in a community.
Antidepressants are used to treat depression, as well as indicated to treat chronic pain and behavioural and psychological symptoms of dementia, including characterisations of Alzheimer’s disease such as insomnia, anxiety and agitation.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe study was based on the register-based MEDALZ cohort built on data derived from community-dwelling people with Alzheimer’s disease in Finland between 2005-2011 and their matched controls.
CalciMedica began Phase I trials for CRAC channel inhibitor
US-based bio-pharmaceutical company CalciMedica initiated the Phase I safety studies in healthy volunteers of a new calcium release-activated calcium (CRAC) channel inhibitor, CM4620, for treating acute pancreatitis.
CRAC channels comprise Orai and regulatory STIM proteins, as well as function to maintain proper levels of calcium in most non-excitable cells.
Acute pancreatitis is a sudden painful inflammation of the pancreas caused due to a mild disorder, but could be very serious if not treated properly.
In worst-case scenarios it could lead to organ failure and sepsis, where the patient needs to stay at the hospital for an extended period of time in the ICU, and substantial morbidity and even mortality are seen.
ThromboGenics began patient enrolment for Phase II trial of THR-317 to treat diabetic macular edema
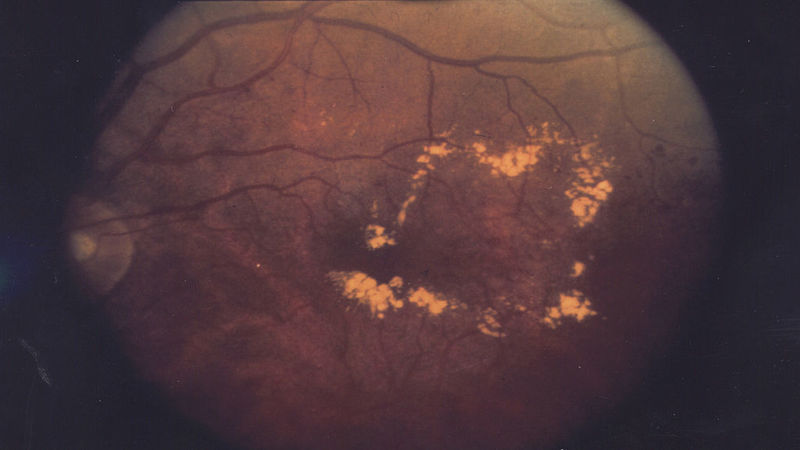
Belgium-based biopharmaceutical company ThromboGenics initiated patient enrolment for its Phase II clinical study of THR-317 to treat diabetic macular edema (DME).
THR-317 (anti-PIGF) has been developed as a recombinant human monoclonal antibody, which targets the receptor-binding site of human placental growth factor (PlGF).
Its mechanism of action involves the reduction of blood vessel leakage, which prevents inflammation in early stage, non-proliferative diabetic retinopathy.
Halozyme Therapeutics reported positive topline results from HALO 202 study of PEGPH20
US-based biotechnology company Halozyme Therapeutics reported positive topline results from a combined analysis of two stages of its Phase II HALO 202 study of PEGPH20 in combination with abraxane and gemcitabine to treat pancreatic cancer.
PEGPH20 is a PEGylated form of the proprietary recombinant human hyaluronidase developed as a potential systemic treatment of tumours that accumulate hyaluronan.
It temporarily degrades HA, which is a dense component of the tumour microenvironment that can accumulate in higher concentrations around certain cancer cells, leading to blood vessel constriction and therapeutic intervention inhibition.
SWOG launched immunotherapy clinical trial to treat rare cancers
SWOG, a part of National Cancer Institute’s National Clinical Trials Network and the NCI Community Oncology Research Programme, launched the DART immunotherapy trial of ipilimumab and nivolumab (Opdivo) to treat rare cancers.
Ipilimumab is a monoclonal antibody drug that inhibits cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), a molecule considered important in relating natural immune responses. This leads to sustaining an active immune response in its attack on cancer cells.
Nivolumab is a PD-1 immune checkpoint inhibitor that binds to the checkpoint receptor PD-1, which is used by the cancer cells to take refuge from the immune system and block the tumour from being exposed to the immune system.
Jazz Pharmaceuticals began enrolment in Phase III trial of defitelio to treat VOD

Ireland-based Jazz Pharmaceuticals began patient enrolment in a Phase III clinical trial of defitelio (defibrotide sodium) against best supportive care (BSC) to address hepatic veno-occlusive disease (VOD) in adult and pediatric patients undergoing hematopoietic stem-cell transplant (HSCT).
Defitelio is an anti-thrombotic, thrombolytic and fibrinolytic polydeoxyribonucleotide, which increases the hydrolysis of fibrin clots by boosting the enzymatic activity of plasmin and minimising expression of adhesion molecules on endothelial cells by releasing prostaglandin 12.
It has been developed by Jazz Pharmaceuticals to treat VOD, also known as sinusoidal obstruction syndrome. The Phase III trial will be conducted as a randomised, open-label, multi-centre trial to compare efficacy of defibrotide against BSC to prevent hepatic VOD.
Prima began dosing patients in Phase IIb trial of IMP321 to treat metastatic breast cancer
Global biotechnology firm Prima BioMed began dosing patients in a Phase IIb clinical trial of its antigen presenting cell (APC) activator, IMP321, for the treatment of metastatic breast cancer.
During the Active Immunotherapy PAClitaxel (AIPAC) trial, half of the 226 patients enrolled for the trial will receive paclitaxel along with a placebo, while the remaining half will receive paclitaxel in conjunction with IMP321.
Once approved by the Dose Escalation Committee, the study will be followed by the use of 30mg dosage level for IMP321.
Sanifit began enrolment in Phase IIb trial of SNF472 to treat cardiovascular calcification
Clinical-stage biopharmaceutical firm Laboratoris Sanifit has enrolled the first patient in CaLIPSO, a Phase IIb study of SNF472 to treat cardiovascular calcification (CVC) in end-stage-renal-disease (ESRD) patients for haemodialysis (HD).
SNF472 is an intravenous drug designed to selectively block the pathological cardiovascular calcification progression, leading to the decrease of cardiovascular events in dialysis patients and in the case of calciphylaxis.
CaLIPSO trial is a 52-week, double-blind, randomised, placebo-controlled study that assesses the effects of SNF472 300 and 600mg doses on the progression of cardiovascular calcification (CVC).
Verastem began dosing in Phase I/II trial of avelumab and defactinib for ovarian cancer
US-based biopharmaceutical firm Verastem began dosing patients in a Phase I/II study of avelumab and defactinib combination to treat patients with advanced ovarian cancer.
Avelumab is an investigational, fully human, anti-PD-L1 IgG1 monoclonal antibody that inhibits PD-L1 interactions and is thought to activate T-cells, as well as the adaptive immune system.
Defactinib is an investigational Focal Adhesion Kinase (FAK) inhibitor designed to modulate the tumour microenvironment, improve anti-tumour immunity, and decrease cancer stem cells.





