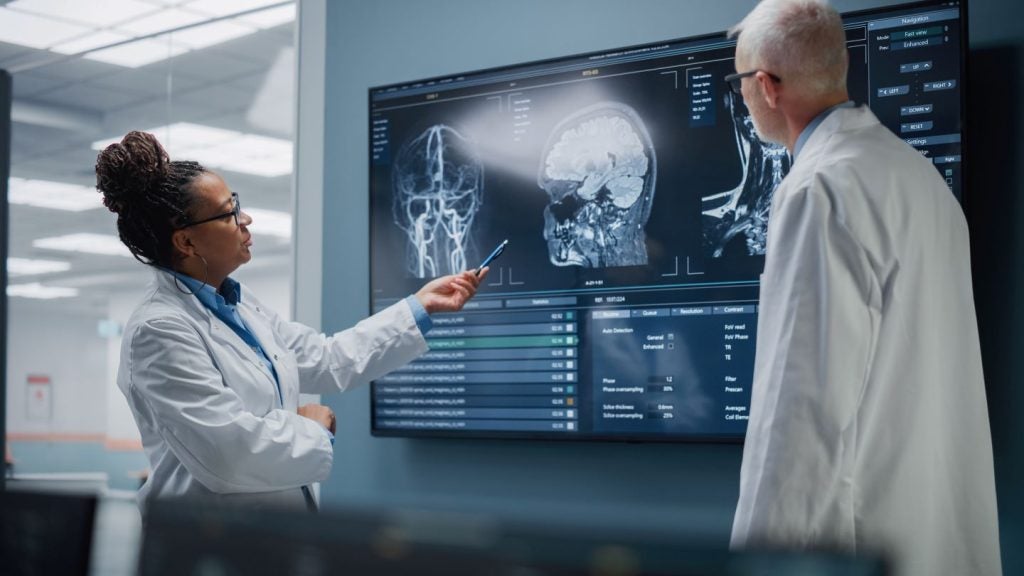
It has been a turbulent few weeks for patients with amyotrophic lateral sclerosis (ALS) after Amylyx Pharmaceuticals pulled its lead drug Relyvrio from the market after a Phase III trial did not meet its primary endpoint.
The drug was pegged to be revolutionary for patients as it was said to slow disease progression, however Phase III data showed no difference between the candidate and placebo.
Despite this setback, chief research officer at the Muscular Dystrophy Association, Dr Sharon Hesterlee, says that there are plenty of novel candidates in the pipeline. “The good news is the pipeline is healthy for ALS, which is important, especially given what has happened with Amylyx’s drug which was disappointing for everyone,” Hesterlee explains.
There are only six FDA approved drugs for ALS – just one of which, riluzole, is a disease modifying therapy, leaving a great need for more treatment options.
Momna Ali, neurology analyst for GlobalData says: “There are 12 assets in their mid-late-stage development (Phase II–III) which are poised to enter the market within the next five years. With numerous pipeline agents that have a diverse mechanism of action under investigation, key opinion leaders interviewed by GlobalData in January 2024 anticipate the treatment landscape to evolve significantly within the next decade.”
GlobalData is the parent company of the Clinical Trials Arena.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataHEALEY Platform trial investigates Calico and AbbVie drug
The HEALEY ALS Platform Trial (NCT04297683) is a perpetual multi-centre, multi-regimen clinical trial evaluating the safety and efficacy of investigational products for the treatment of ALS.
Regimen F is a Phase II/III trial (NCT05740813) which will evaluate the safety and efficacy of a single study drug, ABBV-CLS-7262, in patients with ALS. ABBV-CLS-7262 has been developed in a partnership between Calico Life Sciences and AbbVie.
Regimen F plans to enrol 300 patients with a primary completion date of September 2024.
ABBV-CLS-7262 is an oral candidate which acts by targeting eukaryotic translation initiation factor 2 subunit beta (EIF2B).
The primary endpoint is change in disease severity as measured by the ALS Functional Rating Scale-Revised (ALSFRS-R) total score at 24 weeks.
Secondary endpoints include muscle strength, respiratory function, and disease progression biomarker at 24 weeks.
“In the last couple of years, investigators have really narrowed in on the role of TDP 43 in ALS,” Hesterlee explains. “It will be very interesting to see if blocking that aggregation would be useful. Until we've done the study we're not going to know, but it's certainly nice to see a drug that is trying this.”
Co-director of Massachusetts General Hospital’s Neurological Clinical Research Institute (NCRI), and co-principal investigator in the HEALEY Platform trial, Dr. Sabrina Paganoni, agrees that the candidate has an interesting target.
“This appears to be a promising drug and a very interesting target. I am hopeful if there are positive signals that the candidate could be explored further.”
AL-S Pharma candidate for both sporadic and familial patients
AL-S Pharma is investigating its candidate AP-101 in a Phase IIa trial (NCT05039099) in patients with familial amyotrophic lateral sclerosis (fALS) and sporadic amyotrophic lateral sclerosis (sALS).
The multicentre, randomised, double-blind, placebo-controlled study is evaluating safety, tolerability, pharmacodynamic (PD) markers, and pharmacokinetics (PK) of AP-101.
The study has a primary completion date in June 2024 with an estimated 63 patients.
AP-101 is an intravenous monoclonal antibody which acts by activating superoxide dismutase-1 (SOD1). Mutations in SOD1 leads to ALS.
The primary endpoints in the study are adverse events and abnormalities in vital signs while secondary endpoints will explore levels of cerebrospinal fluid (CSF), changes in neurofilament light chain and phospho-neurofilament heavy chain levels in CSF and plasma.

“It’s a bit of a different way of going after it. It's always good to try these pathways in different ways,” Hesterlee says.
RAPA Therapeutics investigates cell therapy
Rapa Therapeutics is running a Phase II/III trial (NCT04220190) of its candidate RAPA-501. The trial is an open-label, non-randomised, multi-centre study evaluating RAPA-501 T cell therapy in patients with ALS.
RAPA-501 is an autologous T cell therapy which protects motor neuron cells from inflammation. The cells are manufactured ex vivo using epigenetic reprogramming to yield a T cell population that is enriched for a dual anti-inflammatory phenotype based on hybrid TREG and Th2 differentiation.
Patients are dosed with up to four infusions six weeks apart over the 30-week study. There are 41 patients planned to be enrolled in the trial which has a primary completion in July 2025.
“There is some evidence that T regulatory cells can be helpful in ALS, but it usually requires some kind of reprogramming,” says Hesterlee. “In this case, they're not doing any gene engineering but exposing the cells to change their expression patterns, and in theory become helpful in fighting the inflammation that you see in ALS. This looks interesting and is worth following to see how it works.”
Clene’s CNMAu-8 could be a ‘sprinkle of gold dust’
Clene’s CNMAu-8 is being investigated in a Phase II trial with a Phase III due to be launched in 2024.
The Phase II trial (NCT05299658) is an open-label extension trial investigating the candidate in patients with early symptomatic ALS on stable background therapy. The oral candidate is administered once daily over a 48-week period. The trial is due to enrol 40 patients. The trial has a completion date in December 2024.
CNMAu-8 is an oral candidate developed with clean-surfaced, catalytically-active gold nanocrystals. The nanocrystalline suspension inhibits the ability of damaging events leading to dysmyelination and negatively regulates myelination and protects the neurons.
“CNM-Au8 has the ability to cross the blood-brain barrier, which allows it to successfully reach and protect neurons in the central nervous system,” Ali says. “Thus far, it has shown to be safe, well-tolerated and most importantly displays survival benefits, more than some other pipeline agents. Clene is all set for a Phase III to be launched in 2024. If it all works out for Clene, it could quite literally be a sprinkle of ‘’gold dust’’ for patients with ALS.”
Paganoni said that Clene’s candidate was investigated as one of the earlier regimens in the HEALEY trial. “It received a good signal in the platform trial and the company is pursuing that. It is definitely one to watch,” Paganoni explains.