Bristol-Myers’ Phase III lung cancer trial of Opdivo meets endpoint
Bristol-Myers Squibb has stopped CheckMate -057, an open-label, randomised Phase III trial, which is evaluating Opdivo (nivolumab) versus docetaxel in previously treated patients with advanced non-squamous non small cell lung cancer (NSCLC).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The trial was stopped early because an assessment conducted by the independent Data Monitoring Committee (DMC) concluded that the trial met its endpoint, showing superior overall survival in patients receiving Opdivo compared to the control arm.
Opdivo is a programmed death-1 (PD-1) immune checkpoint inhibitor, which has secured approval from the US Food and Drug Administration (FDA) as a monotherapy in two cancer indications.
Merck and Pfizer begin Phase III lung cancer trial of avelumab
Merck and Pfizer have started an international Phase III trial (EMR 100070-004) of its investigational cancer immunotherapy avelumab in patients with stage IIIb/IV non-small cell lung cancer (NSCLC).
The trial is designed to evaluate the efficacy and safety of avelumab compared with docetaxel in these patients who have experienced disease progression after receiving a prior platinum-containing doublet therapy.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAround 650 patients will be enrolled in the trial across 290 sites in more than 30 countries in North America, South America, Asia, Africa and Europe.
AbbVie presents Phase IIIb Ruby-I data of hepatitis C treatment Viekirax+Exviera
Biopharmaceutical firm AbbVie has unveiled the late-breaking and preliminary data of its Phase IIIb Ruby-I study with Viekirax+Exviera in chronic hepatitis c patients with renal impairment.
Viekirax is the fixed-dose combination of paritaprevir 150mg (NS3/4A protease inhibitor) and ritonavir 100mg with ombitasvir 25mg(NS5A inhibitor), while Exviera consists of dasabuvir 250mg (non-nucleoside NS5B polymerase inhibitor).
Ruby-I is an ongoing, multi-centre and open-label Phase IIIb study with two cohorts that assesses the safety and efficacy of 12 or 24 weeks of treatment with Viekirax+Exviera with or without ribavirin.
Teva and Active Biotech enrol first patient in Laquinimod Phase II trial for PPMS
Israeli drugmaker Teva and Swedish pharmaceutical firm Active Biotech enrolled the first patient in a Phase II trial (ARPEGGIO) of laquinimod, an investigational, oral, immune modulator, to treat primary progressive multiple sclerosis (PPMS).
Laquinimod is a once-daily oral, CNS-active immunomodulator with a new mechanism of action under development to treat relapsing-remitting MS (RRMS), progressive MS and Huntington’s disease (HD).
Around 375 patients in the US, Canada and Europe will be enrolled in the multinational, multicentre, randomised, double-blind, parallel-group, placebo-controlled ARPEGGIO trial, which will evaluate the once-daily, oral laquinimod 0.6mg or 1.5mg/day in PPMS patients.
OncoMed begins dosing in Phase II pancreatic cancer trial of demcizumab
US-based OncoMed Pharmaceuticals started dosing in the randomised, double-blind, placebo-controlled Phase II clinical trial (YOSEMITE) of demcizumab (anti-DLL4, OMP-21M18) in patients with first-line metastatic pancreatic cancer.
Demcizumab is a humanised monoclonal antibody that inhibits Delta-Like Ligand 4 (DLL4) in the Notch signaling pathway.
The YOSEMITE trial will compare the efficacy and safety of demcizumab combined with standard of care Abraxane (paclitaxel protein-bound particles for injectable suspension) (albumin bound) plus gemcitabine in patients with first-line metastatic pancreatic cancer.
Merck and NewLink begin Phase II/III trial of Ebola vaccine candidate in Sierra Leone
Merck and NewLink Genetics initiated the third, late-stage, clinical trial of their Ebola vaccine candidate, rVSV-ZEBOV-GP (V920), in Sierra Leone.
Originally developed by the Public Health Agency of Canada’s National Microbiology Laboratory, the Ebola vaccine candidate was licenced to NewLink Genetics in 2010.
The rVSV-ZEBOV-GP vaccine candidate is currently included in three large-scale clinical trials currently underway in West Africa.
Apart from the trial in Sierra Leone, other trials include the Partnership for Research on Ebola Vaccines in Liberia (PREVAIL) and a Phase III trial in Guinea.
Tyrogenex begins Phase II trial of oral treatment with X-82 to treat wet AMD
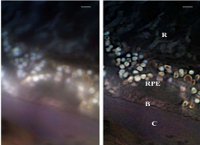
US-based Tyrogenex started a Phase II trial (Apex) of its orally administered drug candidate, X-82, for the treatment of wet age-related macular degeneration (AMD) in previously treated Eylea patients.
X-82 is a dual inhibitor of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) being developed to treat wet AMD and solid tumours.
Around 132 patients are expected to be enrolled in the trial, which is designed to evaluate the safety and efficacy of X-82 in the prevention of vision loss due to wet AMD.
Dermira begins dosing in Phase IIb trial of DRM01 to treat acne vulgaris
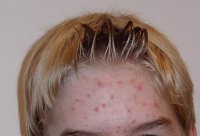
US-based biopharmaceutical firm Dermira dosed its first patient in a Phase IIb dose-ranging trial of DRM01, a new, topical, small-molecule sebum inhibitor, to treat facial acne vulgaris.
Approximately 400 adult patients with moderate-to-severe facial acne vulgaris will be included in the trial and will be randomised into five separate arms evaluating different DRM01 dosing regimens compared to vehicle.
In the trial, 300 patients will receive DRM01, while the remaining 100 will receive vehicle. The company intends to report topline data from the Phase IIb trial in the first half of 2016.





