GSK and MMV initiate Phase III programme for development of malaria drug
GlaxoSmithKline (GSK) and Medicines for Malaria Venture (MMV) initiated a Phase III global programme to assess the efficacy and safety of tafenoquine, an investigational medicine, being developed for the treatment and relapse prevention (radical cure) of Plasmodium vivax (P vivax) malaria.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The Phase III programme includes two randomised, double-blind treatment trials to investigate tafenoquine in adult patients with P vivax malaria. The DETECTIVE study (TAF112582) is designed to assess the efficacy, safety and tolerability of tafenoquine as a radical cure for P vivax malaria, co-administered with chloroquine, a blood stage anti-malarial treatment.
The second trial called GATHER (TAF116564) aims to evaluate the incidence of haemolysis and safety and efficacy of tafenoquine compared with primaquine, the only approved treatment currently available for the radical cure of the disease.
Novartis’s Phase IV trial of Onbrez Breezhaler in COPD patients meets primary objective
Novartis reported positive results from the Phase IV INSTEAD switch trial of Onbrez Breezhaler carried out in patients with chronic obstructive pulmonary disease (COPD).
The company said that the trial met its primary objective and the once-daily Onbrez Breezhaler (indacaterol) 150mcg showed non-inferiority in lung function at week 12 to twice-daily Seretide (salmeterol/fluticasone propionate (SFC)) 50/500mcg in patients with moderate COPD who had experienced no exacerbations in the previous year.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe global, randomised, double-blind, parallel-group, 26-week INSTEAD trial also demonstrated similar symptomatic benefits in terms of shortness of breath and health status after 12 and 26 weeks in patients treated with Onbrez Breezhaler compared to those on SFC.
BioLineRx set to begin investigator-initiated Phase I/II trial of leukaemia drug
Israeli clinical-stage biopharmaceutical firm BioLineRx is starting an investigator-initiated Phase I/II clinical trial of its clinical-stage drug candidate BL-8040 for treatment of patients with chronic myeloid leukaemia (CML), a cancer of white blood cells.
The trial will be carried out by professor Arnon Nagler, director of the Hematology Division and Bone Marrow Transplantation Center at Sheba Medical Center, Israel.
Currently, the company is developing BL-8040 in a Phase II trial for treating acute myeloid leukaemia (AML), and in a Phase I trial for stem cell mobilisation, as a pre-treatment for stem cell transplantation.
Pfizer releases positive top-line results from two Phase III trials of Psoriasis drug tofacitinib
Pfizer released top-line results from two major Phase III trials from the Oral treatment Psoriasis Trials (OPT) Programme, OPT Pivotal #1 (A3921078) and OPT Pivotal #2 (A3921079), of tofacitinib, the first in a new class of medicines, to treat patients with plaque psoriasis.
The trials evaluated the efficacy and safety of tofacitinib, an oral Janus kinase (JAK) inhibitor, for treatment of moderate-to-severe plaque psoriasis.
Both the trials showed that tofacitinib, as a 5mg or a 10mg dose taken as a pill twice-daily, met the primary efficacy endpoints of statistically significant superiority over placebo at week 16.
Cubist Pharma files new drug application with USFDA for ceftolozane/tazobactam
Cubist Pharmaceuticals filed a new drug application (NDA) with the US Food and Drug Administration (FDA) for its investigational antibiotic, ceftolozane/tazobactam for the treatment of complicated urinary tract infections (cUTI) and complicated intra-abdominal infections (cIAI).
The ceftolozane/tazobactam is an antibiotic candidate is being developed for the treatment of certain Gram-negative infections. The NDA submission is based on positive results from its pivotal Phase III clinical trials in cUTI and cIAI.
Ceftolozane/tazobactam, in combination with metronidazole, was studied against meropenen, ceftolozane/tazobactam in cIAI in a pivotal Phase III clinical trial and against levofloxacin in cUTI in a pivotal Phase III clinical trial.
VentiRx Pharma completes enrolment in Phase II study of ovarian cancer drug
US-based VentiRx Pharmaceuticals completed the subject enrollment in GOG-3003, a Phase II study of VTX-2337 in combination with pegylated liposomal doxorubicin (PLD) in patients with recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancer who have failed prior platinum-based chemotherapy.
Being conducted in collaboration with the Gynecologic Oncology Group (GOG) Partners Program, GOG-3003 is one of the two Phase II clinical trials of VTX-2337 currently underway. More than 290 patients at in excess of 85 institutions throughout North America have been randomised in the Phase II GOG-3003 study.
In this placebo-controlled study, women were randomised to receive PLD plus VTX-2337 or PLD plus placebo and the primary endpoint of the study is overall survival.
Abbvie starts Phase III trial of lung cancer drug veliparib
US-based biopharmaceutical firm AbbVie started a global Phase III clinical trial of an investigational oral poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitor ‘veliparib (ABT-888)’ in patients with previously untreated locally advanced or metastatic squamous non-small cell lung cancer (NSCLC).
The company said that PARP is a naturally occurring enzyme in the body that repairs damage to DNA, as well as contributes to chemotherapy resistance in cancer cells. The trial will assess the efficacy and safety of veliparib as an addition to standard chemotherapy in previously-untreated patients.
Around 900 people will be enrolled in the trial, which will compare patients randomised to receive either the standard chemotherapies of carboplatin and paclitaxel with the addition of veliparib, versus patients receiving carboplatin and paclitaxel with the addition of placebo.
Merck reports positive Phase II trial results of chronic hepatitis combination therapy
Merck reported interim results from the ongoing C-WORTHy study, a multi-arm Phase II clinical trial assessing the efficacy and safety of an all-oral, once-daily regimen combining MK-5172 and MK-8742 in patients with chronic HCV Genotype 1 infection (GT1).
The randomised, dose-responsive, parallel-group, multiple-site, double-blind C-WORTHy trial is comparing different patient populations exposed to different durations of treatment of MK-5172 (100mg once-daily) in combination with MK-8742 (50mg once-daily) with or without ribavirin (RBV) in subjects with chronic HCV infection.
MK-5172 is an investigational hepatitis C virus (HCV) NS3/4A protease inhibitor, while MK-8742 is an investigational HCV NS5A replication complex inhibitor.
Critical Pharma announces start of first Phase I study of nano-enabled intranasal teriparatide
UK-based biotechnology firm Critical Pharmaceuticals announced the start of the first Phase I clinical trial of a nano-enabled intranasal teriparatide product in healthy post-menopausal women for the treatment of osteoporosis.
Supported by the University of Nottingham, the trial is being carried out at Nottingham University Hospitals NHS Trust. The new nasal spray will provide patients an easier method of taking the drug teriparatide, which is a highly effective treatment for osteoporosis but which needs to be injected every day.
The nano-enabled nasal spray uses Critical’s CriticalSorb technology to deliver teriparatide in a way that is not only easy for patients to take but also makes it more effective by providing optimal drug levels in the body.
Questcor reports positive clinical trial results of Acthar in nephrotic syndrome patients
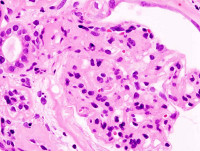
US-based biopharmaceutical firm Questcor Pharmaceuticals reported results from an investigator-initiated clinical trial evaluating the dosing and effectiveness of HP Acthar Gel (repository corticotropin) in 20 patients with nephrotic syndrome due to idiopathic membranous nephropathy (iMN).
Carried out at two major academic research centres, Mayo Clinic and the University of Toronto, the trial enrolled patients with biopsy proven iMN who were randomised 1:1 to receive Acthar at a dose of either 40 or 80 units administered twice weekly following an initial induction period.
Results of the trial showed that Acthar can be a potentially useful therapy for inducing remission of proteinuria in patients suffering from nephrotic syndrome secondary to iMN.





