

Cancer Research UK began new trial for advanced cancer drug
Cancer Research UK has initiated a clinical trial of LY3143921 hydrate at four clinical sites in the country to treat patients with advanced solid tumours.
Originally discovered by Eli Lilly Company, LY3143921 hydrate is being developed as an inhibitor of Cdc7 protein.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Cancers with a fault in their p53 gene such as metastatic bowel, squamous non-small-cell lung and high-grade serous ovarian cancers are expected to be sensitive to Cdc7 inhibition.
Novartis and MMV initiated Phase IIb trial of KAF156 for malaria in Africa
Novartis and Medicines for Malaria Venture (MMV) initiated a Phase IIb clinical trial of KAF156 to treat multidrug-resistant malaria in Africa.
KAF156 belongs to the imidazolopiperazines class of antimalarial compounds and is believed to have the potential to clear infection and also prevent the transmission of the malaria parasite.
The Phase IIb trial is designed to evaluate the efficacy of KAF156 in combination with a new and improved formulation of an existing antimalarial drug called lumefantrine.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataMallinckrodt’s Phase IV registry for PPHN medicine started enrolment
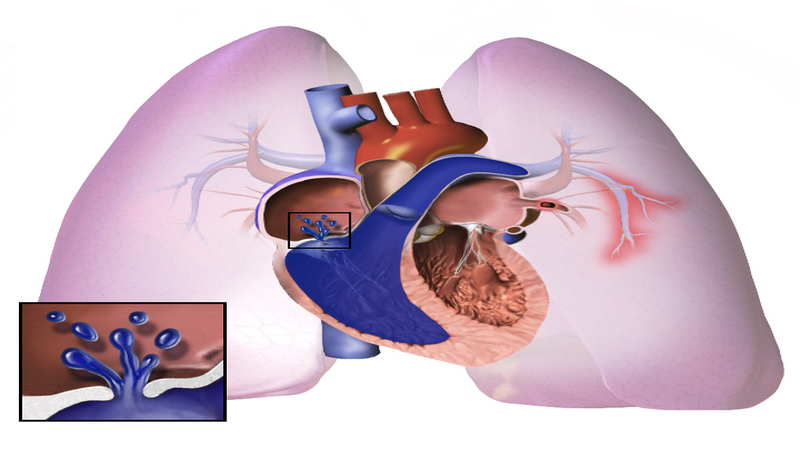
Mallinckrodt Pharmaceuticals started patient enrolment in a Phase IV registry (PaTTerN) to evaluate INOMAX (nitric oxide) gas inhalation to treat persistent pulmonary hypertension of the newborn (PPHN).
The trial will be conducted in premature neonates whose gestational age is less than 34 weeks and in term, near-term neonates with a gestational age of more than 34 weeks.
INOMAX is indicated for oxygenation improvement and to minimise extracorporeal membrane oxygenation need in term and near-term neonates with pulmonary hypertension (PH)-associated hypoxic respiratory failure (HRF), in combination with ventilatory support.
Eli Lilly’s lasmiditan met Phase III trial endpoints for migraine
Eli Lilly and Company reported positive results from the Phase III SPARTAN clinical trial of lasmiditan for the acute treatment of migraines.
Lasmiditan is an investigational molecule designed to specifically target 5-HT1F receptors that are expressed in the trigeminal pathway, without the need for vasoconstrictor activity.
The results showed that lasmiditan met the primary endpoint with a greater proportion of patients being migraine-free, as well as the secondary endpoint with a statistically significant percentage of subjects experiencing relief from their most bothersome symptom two hours after the first dose.
UCL research indicated use of diabetes drug in Parkinson’s disease
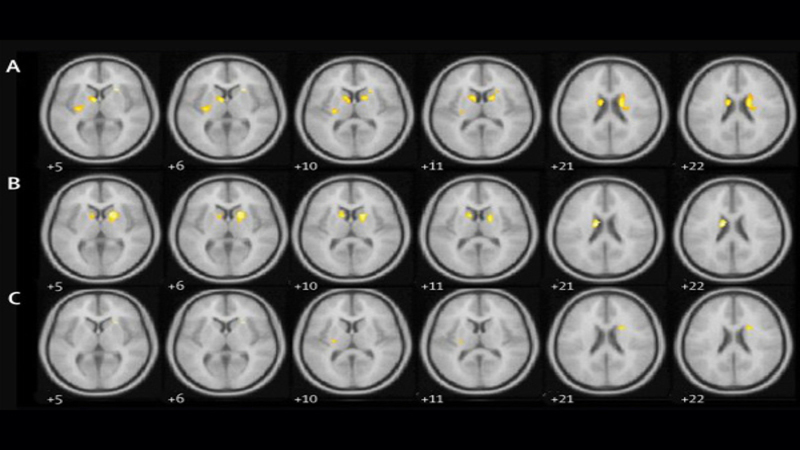
University College London (UCL) carried out a study that indicated the potential of a common diabetes drug called exenatide as a disease-modifying therapy for treating Parkinson’s disease.
Funded by the Michael J Fox Foundation for Parkinson’s Research (MJFF), the study involved administration of 60 Parkinson’s disease patients with exenatide once every week over one year.
The patients were monitored by the researchers at the National Hospital for Neurology and Neurosurgery (NHNN).
Vyriad and Sanford Health began Phase I viro-immunotherapy trial
US-based biopharmaceutical firm Vyriad initiated a clinical trial at integrated health system Sanford Health to evaluate a genetically engineered virus for destroying therapy-resistant tumours.
During the trial, metastatic solid tumour patients aged 18 and above who have not responded to standard treatments will be administered with an oncolytic virus called vesicular stomatitis virus (VSV) into the tumour.
The virus is intended to grow in cancer cells, destroy them and later spread to other cancer affected sites, as well as recruit the immune system to the site to trigger an immune response.
Cyclodextrin slowed NPC progression in new Washington University trial

A new clinical trial conducted by Washington University School of Medicine in St Louis and the National Institutes of Health (NIH) in the US showed that a drug called cyclodextrin slows progression of Niemann-Pick type C (NPC) disease.
NPC is a neurodegenerative condition that causes a cholesterol build up in neurons and results in the gradual loss of brain function, while cyclodextrin is a type of sugar molecule that is believed to act by releasing the trapped cholesterol.
The researchers carried out a combined Phase I/II clinical trial in 14 subjects aged between four and 23 years with neurological symptoms.
Zealand partner Boehringer began Phase I trials for two obesity and / or diabetes drugs
Zealand Pharma announced the launch of two Phase I clinical trials by its partner Boehringer Ingelheim to investigate a glucagon / GLP-1 agonist and an amylin analogue for the treatment of obesity and / or type 2 diabetes.
With a dual-acting mechanism, the glucagon / GLP-1 agonist triggers GLP-1 and glucagon receptors and is expected to provide better blood sugar and weight loss control.
The compound is partly based on the effects of a natural gut hormone called oxyntomodulin that is known to minimise food intake and improve energy expenditure.
Pfizer and AFT initiated Phase III trial of HER2+ breast cancer drug
Pfizer and the Alliance Foundation Trials partnered with six international cancer research groups to initiate a Phase III clinical trial (PATINA) of palbociclib (IBRANCE) in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer.
Palbociclib is being developed as an inhibitor of cyclin-dependent kinases (CDK) 4 and 6 that are involved in regulation of the cell cycle associated with cellular progression.
The international, randomised, open-label, multi-centre Phase III trial will compare the combination of palbociclib, anti-HER2 therapy and endocrine therapy with standard therapy as a first-line treatment in a total of 500 patients.
Researchers demonstrated use of breast cancer drug to shrink brain tumours
Researchers from the University of Queensland’s Institute for Molecular Bioscience in Australia and the Fred Hutchinson Cancer Research Centre in the US conducted a clinical trial to evaluate a previously approved breast cancer drug palbociclib to treat brain tumours.
The trial enrolled children with a common type of brain tumour called medulloblastoma and demonstrated that palbociclib could shrink the tumours.
University of Queensland professor Brandon Wainwright said: “Clearly, we need new therapies that increase survival of young patients and reduce the side effects they suffer, such as delays in brain development, growth problems and increased risk of other cancers.”



