

Gradalis began dosing in Phase I breast cancer trial of Vigil EATCs and durvalumab
US-based clinical-stage biopharmaceutical company Gradalis dosed the first patient in its pilot Phase I trial of a combination of Vigil Engineered Autologous Tumour Cells (EATCs) and durvalumab to treat advanced breast cancer.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Vigil is an investigational, cellular immunotherapy technology based on a combination of genetic engineering concepts with the science of immuno-oncology to induce an immune response to cancer cells.
A patient's tumour cells are engineered with a plasmid carrying the gene vector for shRNA Furin and GMCSF, to issue a systemic, T-cell directed immune response administered to the patient through intradermal injections.
Biothera and Merck conducted combination cancer immunotherapy trials
US-based biotechnology company Biothera collaborated with Merck to expand the ongoing clinical programme of Merck’s KEYTRUDA and Biothera’s Imprime PGG to treat advanced melanoma or metastatic triple negative breast cancer (TNBC).
Humanised monoclonal antibody Keytruda boosts the body's immunity system to detect and fight tumour cells.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt resists PD-1 from interacting with its ligands, PD-L1 and PD-L2, subsequently activating T-lymphocytes that may affect both tumour and healthy cells.
Themis Bioscience initiated Phase II trial of Chikungunya vaccine
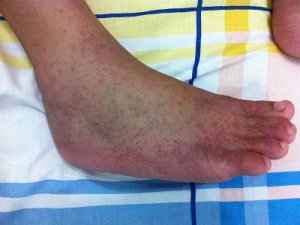
Europe-based Themis Bioscience began the Phase II clinical trial of its prophylactic vaccine candidate against Chikungunya fever by vaccinating the first patient.
The vaccine is based on the company’s patented Themaxyn platform, which uses a standard measles virus vaccine as a vector, developed at the Institut Pasteur in Paris.
Gene coding for selected antigens derived from the Chikungunya virus have been included in the genome of the measles vaccine delivering those new antigens into cells. This will result in a specific immune response against the Chikungunya virus.
Inovio Pharmaceuticals began second clinical trial of Zika vaccine in Puerto Rico
US-based Inovio Pharmaceuticals began its second clinical study of its Zika vaccine, GLS-5700 in Puerto Rico, which has declared the Zika virus outbreak as a public health emergency.
The company is developing its preventive Zika vaccine, GLS-5700, in collaboration with GeneOne Life Science and other academic collaborators from the US and Canada.
GLS-5700 has been designed to offer broad protective antibody and trigger therapeutic T-cell responses against a range of Middle East respiratory syndrome (MERS) viruses, including the Zika virus.
Novartis reported positive Phase III EXPAND trial results of BAF312 to treat SPMS
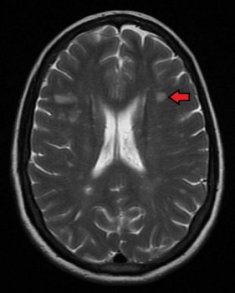
Novartis reported positive Phase III EXPAND trial results of BAF312 (siponimod) to treat secondary progressive multiple sclerosis (SPMS).
BAF312 was developed as a selective modulator of specific types of the sphingosine-1-phosphate (S1P) receptor.
Found on the surface of specific cells residing in the central nervous system (CNS), the S1P receptor is considered responsible for causing CNS damage resulting to loss of function in secondary progressive MS (SPMS).
Children’s Hospital Los Angeles conducted Phase I study of Durvalumab to treat pediatric cancer
The Children’s Centre for Cancer and Blood Diseases (CCCBD) at Children’s Hospital Los Angeles conducted the first-in-pediatrics Phase I study of Durvalumab to treat children with solid tumours, lymphoma and central nervous system tumours.
The aim of this Phase 1 trial was to test the safety, tolerability and metabolism of the drug Durvalumab in pediatric patients.
Durvalumab is also being investigated in several kinds of adult cancer and is known as a checkpoint inhibitor.
NIH initiated clinical trial of Zika virus vaccine
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), launched a VRC 319 clinical trial of a vaccine to prevent Zika infection.
The NIAID Zika virus investigational DNA vaccine consists of a small, circular piece of DNA known as plasmid, designed to contain genes that code for proteins of the Zika virus.
After the vaccine is injected into the arm muscle, the cells analyse the gene and make Zika virus proteins, which self-assemble into virus-like particles.
Eli Lilly recommended to conduct Monarch II Phase III trial of abemaciclib to treat breast cancer
Eli Lilly and Company was recommended to continue its MONARCH II trial without any modification to compare a combination of abemaciclib and fulvestrant against placebo with fulvestrant to treat locally advanced or metastatic breast cancer.
The recommendation was made by an independent Data Monitoring Committee (DMC) after completing a pre-planned interim analysis for MONARCH II, which suggested that the trial did not fulfill its interim efficacy criteria.
Abemaciclib (LY2835219) is an investigational, oral-cell cycle inhibitor that was developed to inhibit cancer cell growth by specifically blocking cyclin-dependent kinases, CDK 4 and CDK 6.
TARIS began Phase Ib trial of TAR-200 to treat bladder cancer
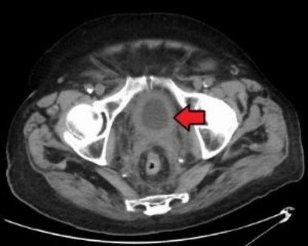
US-based, therapeutically focused urology company TARIS Biomedical began the Phase Ib clinical trial of TAR-200 to treat non-muscle-invasive bladder cancer (NMIBC).
TAR-200, also known as gemcitabine releasing intravesical system (GemRIS), is a drug-device combination product based on the TARIS system, designed to continuously release gemcitabine into the bladder for seven days.
Gemcitabine is designed to treat multiple cancers individually or while combined with other chemotherapeutic drugs.
Pfizer reported positive top-line results for Phase III OCTAVE Sustain trial of XELJANZ to treat UC
US-based pharmaceutical company Pfizer reported positive top-line results from the Phase III oral clinical trials for tofacitinib in ulcerative colitis (OCTAVE) Sustain trial of XELJANZ, to treat moderately to severely active ulcerative colitis (UC).
XELJANZ (tofacitinib citrate) is a prescription medicine inhibiting Janus kinase (JAK), an enzyme generating cytokine-mediated signals through the JAK-STAT pathway.
The randomised, double-blind, placebo-controlled, parallel group, multi-centre Phase III OCTAVE Sustain trial has been designed to evaluate the safety and efficacy of orally administered tofacitinib as a maintenance therapy to treat adult patients with moderately to severely active UC.





