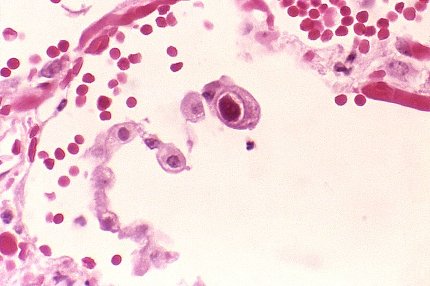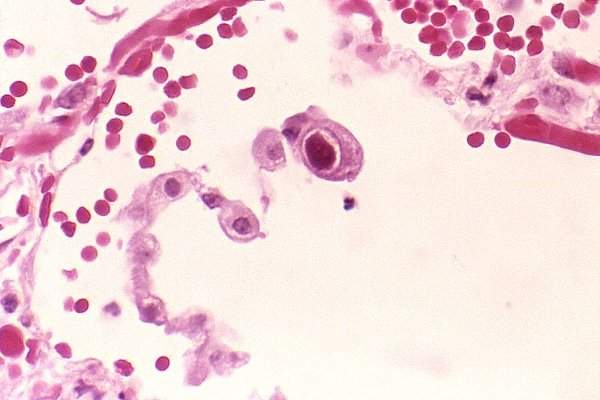
Merck begins Phase III letermovir trial for prevention of CMV infection
US-based pharmaceutical firm Merck enrolled the first patient in a global Phase III clinical trial of letermovir (MK-8228), an investigational antiviral agent, for the prevention of the cytomegalovirus (CMV) infection in high-risk bone marrow transplant patients.
The trial will assess the efficacy and safety of letermovir for the prevention of clinically-significant CMV infections in adults aged 18 years and older who are CMV-seropositive recipients of allogeneic hematopoietic stem cell transplants.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Letermovir is being developed for the prevention of human CMV infection and has secured orphan product designation from the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA).
Janssen Biotech to initiate Phase III trial of multiple myeloma treatment in Q4 2014
Genmab partner Janssen Biotech announced plans to initiate a new Phase III trial of daratumumab to treat patients with multiple myeloma in the fourth quarter of 2014.
The trial will compare daratumumab in combination with bortezomib, melphalan and prednisone to bortezomib, melphalan and prednisone alone as frontline treatment for patients who are not considered candidates for stem cell transplantation (SCT).
The Phase III trial will include appoximately 700 newly diagnosed, chemotherapy naive multiple myeloma patients ineligible for stem cell transplantation (SCT).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataBMS and Pfizer initiate Phase IV Eliquis trial to treat non-valvular atrial fibrillation
Bristol-Myers Squibb (BMS) and Pfizer initiated patient enrolment in a Phase IV clinical trial called EMANATE, which is evaluating the effectiveness and safety of Eliquis in patients with nonvalvular atrial fibrillation (NVAF) undergoing cardioversion.
At present, Eliquis is approved to reduce the risk of stroke and systemic embolism in NVAF patients. Cardioversion is a commonly used, effective method of converting atrial fibrillation to a normal rhythm, allowing the heart to pump more effectively.
In the randomised, open-label EMANATE (Eliquis evaluated in acute cardioversion compared to usual treatments for anticoagulation in subjects with NVAF) trial, the safety and efficacy of Eliquis will be evaluated compared to the usual care started in patients with NVAF expected to undergo cardioversion after short-term anticoagulation, in a clinical practice setting.
Merck Serono begins MSB0010718C Phase II trial for carcinoma treatment
Merck’s biopharmaceutical division Merck Serono started an international Phase II trial designed to evaluate the efficacy and safety of MSB0010718C in patients with metastatic Merkel cell carcinoma (mMCC), a rare and aggressive type of skin tumour.
MSB0010718C is an investigational fully human IgG1 monoclonal antibody that binds to programmed death-ligand 1 (PD-L1).
The multicentre, single-arm, open-label Phase II trial is being carried out in patients with mMCC who have previously received one line of chemotherapy.
Bayer and Onyx’s sorafenib Phase III breast cancer trial fails to meet primary endpoint
Bayer HealthCare Pharmaceuticals and Onyx Pharmaceuticals reported results from an investigational Phase III trial of sorafenib (Nexavar) tablets plus capecitabine in patients with advanced breast cancer.
The Phase III trial, called RESILIENCE, did not meet its primary endpoint of improving progression-free survival (PFS).
During the trial, the efficacy and safety of sorafenib was evaluated in combination with capecitabine, an oral chemotherapeutic agent, compared to a placebo plus capecitabine.
GSK and Theravance begin Phase III trial of triple combination treatment in COPD patients
GlaxoSmithKline and Theravance initiated a global Phase III trial to assess the safety and efficacy of a closed triple combination in patients with chronic obstructive pulmonary disease (COPD).
IMPACT is the first Phase III trial in a programme to assess a once-daily closed triple combination treatment of an inhaled corticosteroid (ICS), a long-acting muscarinic antagonist (LAMA) and a long-acting beta2-adrenergic agonist in COPD patients.
The triple combination is comprised of FF (fluticasone furoate, an ICS), UMEC (umeclidinium, a LAMA) and VI (vilanterol, a LABA).
Sanofi Pasteur reports positive Phase III results from dengue vaccine trial
Sanofi Pasteur reported the results of its first randomised, observer-blind, placebo-controlled multicentre Phase III dengue vaccine efficacy trial carried out in five countries in Asia.
The results show overall efficacy against symptomatic dengue of 56.5% in children aged two to 14 years old after a three-dose vaccination schedule.
Akashi reports positive trial results and gets fast-track status for DMD drug HT-100
US-based biopharmaceutical firm Akashi Therapeutics reported preliminary positive clinical results from a Phase Ib/IIa trial of its drug candidate, HT-100 (delayed-release halofuginone), for treatment of Duchenne muscular dystrophy (DMD).
HT-100 is an orally available, small molecule developed to reduce fibrosis and inflammation and promote healthy muscle regeneration in boys with DMD.
The preliminary data for HT-100 on the first DMD patient groups show promising signs of biological activity and a favourable safety profile to date in this ongoing Phase Ib/IIa multi-centre clinical programme to assess the safety and tolerability of increasing doses of the drug candidate and evaluate trends in a range of exploratory biomarkers and efficacy endpoints.