
Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.

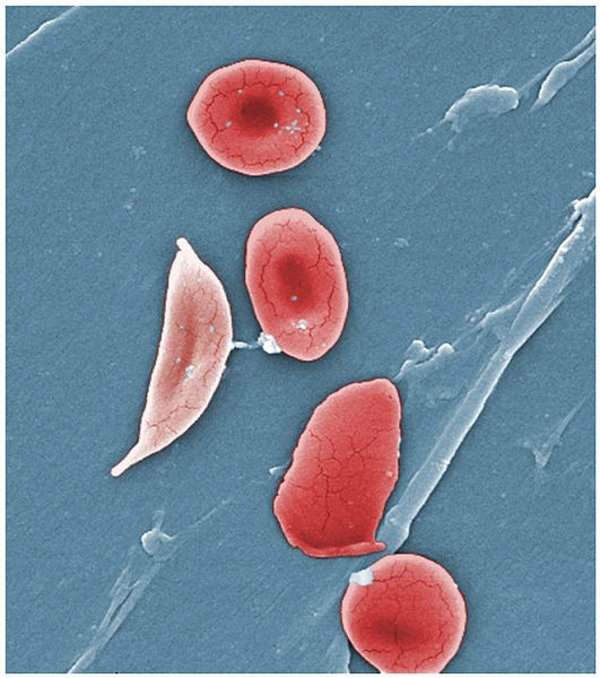
Pfizer has enrolled the first patient in its Phase III clinical trial (Reset) of rivipansel, an investigational pan-selectin inhibitor, to treat vaso-occlusive crisis (VOC) in patients hospitalised with sickle cell disease (SCD) who are six years of age or older.
Around 350 people with sickle cell disease will be enrolled in the Rivipansel: Evaluating Safety, Efficacy and Time to Discharge (Reset) trial, which will evaluate the efficacy and safety of rivipansel.
The trial's primary endpoint will be time to readiness-for- discharge, while key secondary endpoints will include time to discharge, cumulative IV opioid consumption and time to discontinuation of IV opioids.
SCD, a rare and debilitating chronic disease, is one of the most prevalent genetic disorders in the US and life expectancy for females and males with this disease is 48 and 42 years, respectively.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSouth Korea commences trial of experimental plasma treatment for MERS
South Korea has reportedly commenced trials of an experimental plasma treatment for Middle East Respiratory Syndrome (MERS), as part of efforts to control the disease in the country.
Earlier, the treatment was proved to have helped tackle diseases such as Ebola.
According to the South Korean health ministry, the two hospitals are being involved in the plasma treatment studies.
Around 19 people have died due to the respiratory disease and approximately 150 have been infected in the country.
NIH's NIAAA initiates clinical trial of Horizant to treat alcohol use disorder
The US National Institutes of Health's (NIH) National Institute on Alcohol Abuse and Alcoholism (NIAAA) has initiated a clinical trial of Horizant (gabapentin enacarbil) extended-release tablets to treat patients with moderate to severe alcohol use disorder (AUD).
NIAAA is working in partnership with XenoPort, a biopharmaceutical company, which will supply gabapentin enacarbil for the trial. A total of 346 patients will be enrolled in this randomised, double-blind, placebo-controlled clinical trial at ten sites across the US.
The trial is designed to evaluate the safety and efficacy of 1,200mg of Horizant, administered in two daily doses of 600mg each, compared with placebo to reduce drinking in subjects who report four or more symptoms of AUD.
Lilly and Immunocore enter clinical trial collaboration for new melanoma treatment
Eli Lilly and Company and Immunocore have entered into an immunotherapy-based clinical trial collaboration to explore the utility of IMCgp100 in combination with galunisertib and merestinib to treat melanoma.
The deal is aimed at identifying combination regimens that provide synergies in efficacy and durability in patients with metastatic cutaneous and uveal melanomas.
IMCgp100 is Immunocore's lead T cell receptor-based investigational therapeutic, while galunisertib (LY2157299) is Lilly's TGF beta R1 kinase inhibitor and merestinib (LY2801653) is a multi-kinase inhibitor.
Amgen reports Phase II interim results of migraine treatment AMG33

US-based Amgen has reported positive interim results from its ongoing Phase II study of human monoclonal antibody, AMG33, to prevent episodic migraine.
AMG 334 is a fully human monoclonal antibody that is being developed to prevent migraine. It targets the calcitonin gene-related peptide (CGRP) receptor, which is expected to transmit signals that can cause incapacitating pain.
The trial is a double-blind and placebo-controlled study, which is assessing the safety and efficacy of AMG 334 for the prevention of episodic migraine.
AstraZeneca and Lilly establish clinical trial collaboration in solid tumours

British-Swedish drugmaker AstraZeneca has established a clinical trial collaboration with Eli Lilly and Company to assess the safety and preliminary efficacy of its investigational anti-PD-L1 immune checkpoint inhibitor MEDI4736 in combination with ramucirumab (Cyramza), Lilly's VEGF Receptor 2 antiangiogenic cancer medicine.
The planned Phase I study will evaluate the combination as a treatment for patients with advanced solid tumours.
Developed by AstraZeneca's global biologics research and development arm MedImmune, the MEDI4736 is an investigational human monoclonal antibody directed against programmed cell death ligand 1 (PD-L1).
AbbVie reports veliparib's Phase II study results for NSCLC
AbbVie has revealed the Phase II study results of the investigational medicine veliparib in patients with non-small cell lung cancer (NSCLC).
The veliparib combined with the chemotherapy regimen carboplatin and paclitaxel demonstrated an improvement in median progression-free survival (PFS) in patients with previously untreated metastatic or advanced NSCLC who are current smokers.
Veliparib is an investigational oral poly is an investigational oral poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitor, which is being assessed to treat various cancer types, including NSCLC.
Astellas, Medivation commence TRUMPET study to assess patients with CRPC
Astellas and Medivation have enrolled first patients in Treatment Registry for Outcomes in CRPC Patients (TRUMPET) study, which is designed to better understand the unique needs and treatment patterns for patients with castration-resistant prostate cancer (CRPC).
The prospective and observational patient registry will enrol and assess 2,000 patients diagnosed with CRPC from urology and oncology sites across the US.
Under the study, the firms will also collect data from the primary caregivers of patients, comprising spouses, family members and friends.





