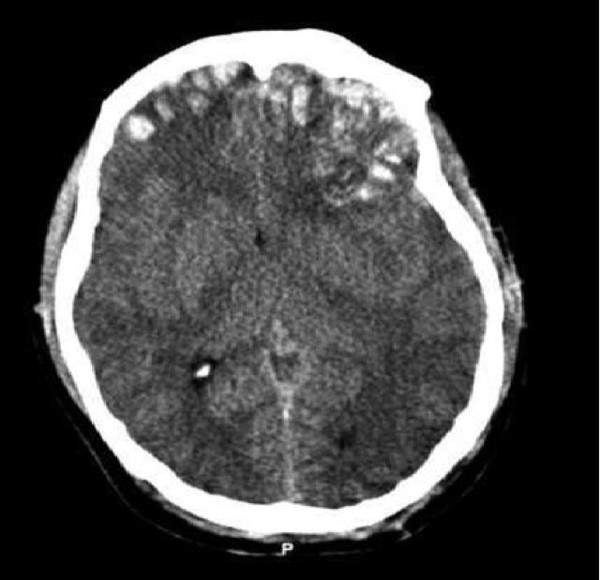
ProMedica and Stemedica planned to begin human stem cell trial for traumatic brain injury
US-based companies ProMedica and Stemedica Cell Technologies (Stemedica), along with the family of hockey legend Gordie Howe, have planned to begin a human stem cell trial to advance the treatment of traumatic brain injury (TBI).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The three-year study, known as Gordie Howe Initiative, will support clinical research and increase awareness about TBI with an initial focus on war veterans, athletes and automobile accident victims.
The first stage of the initiative will include a US-based Phase IIa clinical trial to evaluate preliminary safety and efficacy of Stemedica’s proprietary allogeneic mesenchymal stem cells (MSCs), in patients with moderate to severe TBI.
Cerulean began dosing in Phase l/ll combination trial of CRLX101 and lynparza
US-based, clinical-stage company Cerulean Pharma dosed the first patient in a Phase l/ll clinical trial of its lead nanoparticle-drug conjugate NDC candidate, CRLX101, in combination with lynparza (olaparib) in patients with advanced solid tumours.
The open-label, single centre trial is being conducted by the National Cancer Institute (NCI), US, along with AstraZeneca and Cerulean.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe trial is led by NCI investigators, Dr Anish Thomas and Dr Yves Pommier.
Akili began enrolment for STARS-ADHD trial to treat children with ADHD
Akili Interactive Labs (Akili) began enrolment for its STARS-ADHD trial, which will evaluate the safety and efficacy of the company’s proprietary platform, Project: EVO, for the treatment of children with Attention Deficit Hyperactivity Disorder (ADHD).
ADHD is a neurological condition marked by inattention and / or hyperactivity-impulsivity, and though about 75% of young children with ADHD receive medication, there is a growing demand for non-pharmacological interventions.
Project: EVO is designed to directly target an individual’s core ability to process multiple streams of information, which has the potential to change specific neural networks and improve attention, inhibition and working memory.
Seattle Genetics began Phase lll combination trial of vadastuximab talirine and azacitidine to treat AML
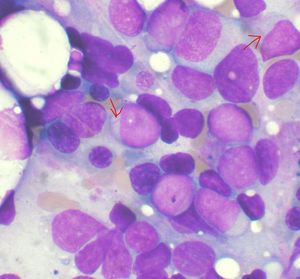
US-based biotechnology company Seattle Genetics began a Phase lll clinical trial evaluating vadastuximab talirine (SGN-CD33A; 33A), in combination with azacitidine (Vidaza) or decitabine (Dacogen) in older patients with newly diagnosed acute myeloid leukaemia (AML).
CASCADE is a randomised, double-blind, placebo-controlled, global clinical trial.
During the trial, patients will be randomised on a 1:1 ratio to be treated with an hypomethylating agent (HMA) plus 33A or an HMA plus placebo.
Tonix reported positive results from Phase ll trial of TNX-102 SL to treat PTSD
Tonix Pharmaceuticals reported positive results from a Phase ll, dose-finding clinical trial of TNX-102 SL (cyclobenzaprine HCl sublingual tablets) in military-related post-traumatic stress disorder (PTSD), AtEase study.
PTSD can develop from witnessing or experiencing a traumatic event or ordeal whereby there was a severe threat of or actual occurrence of grave physical harm.
TNX-102 SL is designed to rapidly provide cyclobenzaprine to the bloodstream through sublingual, under the tongue, absorption and to bypass first-pass hepatic metabolism.
Merrimack began Phase l combination trial of MM-151 and onivyde to treat metastatic colorectal cancer
Merrimack Pharmaceuticals and Baxalta began a Phase l clinical trial of MM-151 in combination with onivyde (irinotecan liposome injection), fluorouracil (5-FU) and leucovorin to treat RAS wild-type, metastatic, colorectal cancer patients.
Merrimack’s MM-151 is an oligoclonal, epidermal growth factor receptor (EGFR) inhibitor that contains three fully-human monoclonal antibodies designed to bind and inhibit signalling of the EGFR.
The drug has previously been tested in a Phase l dose-escalation clinical trial in patients with advanced solid tumours.
GSK and Innoviva reported positive results from Phase IIIb trial of relvar ellipta to treat COPD

GlaxoSmithKline (GSK) and Innoviva reported positive results from a Phase IIIb trial to compare the effectiveness and safety of relvar ellipta (FF / VI) 100mcg / 25mcg with existing usual care to treat chronic obstructive pulmonary disease (COPD).
A total of 2,802 patients with COPD were enrolled in this multi-centre, open label, randomised controlled Phase IIIb trial, called Salford Lung Study (SLS).
COPD is a disease of the lungs that involves chronic bronchitis, emphysema or both.
Nordic Nanovector began enrolment in expanded study of betalutin to treat NHL
Norway-based biotechnology company Nordic Nanovector enrolled its first patient in arm three of expanded Phase l/ll study, Lymrit 37-01, of betalutin to treat non-Hodgkin’s lymphoma (NHL).
Betalutin is a new anti-CD37 that aims antibody radionuclide conjugate in development for the treatment of major types of NHL, including follicular lymphoma (FL).
The arm three of the trial is designed to investigate the safety and efficacy of Betalutin in up to 12 patients with relapsed FL pre-dosed and standard anti-CD20 immunotherapy (rituximab) on day 0, a few hours prior to the administration of Betalutin.
Gelesis reported positive safety data from first-in-human study of Gelesis200 to treat type 2 diabetes
Biotechnology company Gelesis reported positive results from a first-in-human study of Gelesis200, the company’s next-generation product designed to treat type 2 diabetes patients.
Gelesis200 is an orally administered capsule containing small hydrogel particles, which seeks to encourage weight loss and improve glycemic control in patients with type 2 diabetes.
The study results showed that Gelesis200 was generally well-tolerated and no serious adverse events (AEs) were reported during the single-centre, randomised, double-blind, placebo-controlled, two-cohort, four-arm, crossover study.
Acucela began Phase ll trial of emixustat to treat PDR
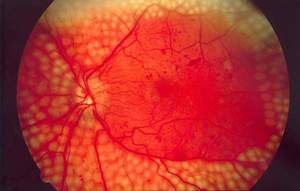
Clinical-stage ophthalmology company Acucela started a Phase ll clinical trial to evaluate the benefits of emixustat hydrochloride (emixustat) to treat proliferative diabetic retinopathy (PDR).
It enrolled the first patient for the randomised, placebo-controlled trial.
The company has already submitted a clinical study protocol to the US Food and Drug Administration (FDA) and has received Institutional Review Board approval to initiate the study.





