
GlaxoSmithKline begins Phase III trial of sirukumab to treat giant cell arteritis
GlaxoSmithKline (GSK) started dosing in a Phase III trial evaluating sirukumab, a human anti-interleukin (IL)-6 monoclonal antibody, to treat patients with giant cell arteritis (GCA).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Sirukumab is an investigational human anti-IL-6 monoclonal antibody. It binds with high affinity to the IL-6 cytokine, a naturally occurring protein believed to play a role in autoimmune conditions.
Sirukumab was in a phase III development for rheumatoid arthritis (RA) and GCA.
Pfizer’s neuropathic pain drug Lyrica fails Phase III trial
US drugmaker Pfizer reported results from its Phase III trial of Lyrica (pregabalin) Capsules CV in adults with chronic post-traumatic peripheral neuropathic pain.
The trial failed to meet the primary efficacy endpoint, which was an overall pain reduction compared with placebo results.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe pain reduction was measured using pain scores from patient diaries.
Seattle begins Phase I/II trial of SGN-CD33A to treat AML
US-based biotechnology firm Seattle Genetics started a Phase I / II clinical trial of SGN-CD33A (vadastuximab talirine), a new antibody-drug conjugate (ADC), to treat patients with relapsed or refractory acute myeloid leukemia (AML).
The trial was designed to evaluate SGN-CD33A monotherapy as a pre-conditioning regimen prior to an allogeneic stem cell transplant (alloSCT), and also as a maintenance therapy following a transplant.
Using the company’s new ADC technology, SGN-CD33A targets CD33, which is expressed on almost all AML cells regardless of subtype, cytogenetic abnormality, or underlying mutations.
Otonomy begins enrolment in US Phase III trial of OTO-104 to treat Meniere’s disease
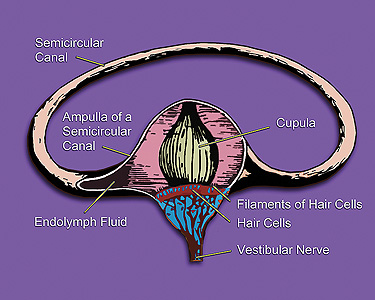
Otonomy started patient enrolment in the US Phase III clinical trial of OTO-104, a sustained-exposure formulation of the steroid dexamethasone, to treat Ménière’s disease.
Also called endolymphatic hydrops, Menière’s disease is a disorder of the inner ear that can affect hearing and balance. It is characterised by acute vertigo attacks, tinnitus, fluctuating hearing loss, and a feeling of aural fullness.
The company noted that a second Phase III trial was expected to be initiated in the European Union (EU) in early 2016.
AbbVie presents SURVEYOR study results for its investigational HCV regimen
Biopharmaceutical company AbbVie presented data from the SURVEYOR studies of ABT-493, an NS3 / 4A protease inhibitor, and ABT-530, an NS5A inhibitor.
ABT-493 is the result of an ongoing partnership between AbbVie and Enanta Pharmaceuticals for HCV protease inhibitors and regimens, including protease inhibitors.
The inhibitor was being developed for use in combination with AbbVie’s other investigational medicines for the treatment of hepatitis C.
Baxalta seeks UK MHRA approval for BAX 826 trial of to treat hemophilia A
US-based Baxalta submitted a clinical trial application (CTA) to the UK Medicines and Healthcare Products Regulatory Agency (MHRA), seeking approval to start a first-in-human clinical trial of BAX 826.
BAX 826 is an investigational, extended half-life recombinant Factor VIII (rFVIII), and a potential treatment for hemophilia A.
The open-label trial was to enrol about 30 patients, and evaluate the safety and efficacy of BAX 826.
PhaseBio begins PE0139 Phase IIa trial to treat hyperglycemia
PhaseBio Pharmaceuticals started a Phase IIa clinical trial of PE0139, a potential treatment for hyperglycemia associated with diabetes.
PE0139 is a fusion of a fully mature, native insulin molecule with the company’s polypeptide biopolymer.
PhaseBio develops treatments for metabolic and cardiopulmonary disorders.
Hutchison MediPharma begins Phase I trial of sulfatinib to treat advanced solid tumours
Hutchison MediPharma (HMP) started a Phase I clinical trial of sulfatinib (HMPL-012) to treat patients with advanced solid tumours.
The US Food and Drug Administration (FDA) had already cleared the investigational new drug (IND) application for sulfatinib.
HMP was planning two Phase III studies for neuroendocrine tumours (NET) and a Phase Ib trial in China for the treatment of thyroid cancer.





