Pharma Technology Focus is free for the iPad. Download our app to read the latest issue and browse our back issues for free

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Russia has been boosting its credentials as a prime location for early-stage clinical trials, with 70% of new drugs registered in Europe in 2015 tested in phase I-II studies in the country. We explore Russia’s growing popularity as a trial location and challenges that remain or foreign companies looking for study sites.
We also find out how new research into the human microbiome could support the development of drugs that mitigate the effects of radical treatments such as chemotherapy on the body, and hear from GlobalData why a concerted global effort is needed to tackle the threat of antimicrobial resistance.
Also in this issue, we find out from Amplexor Life Sciences how the life sciences sector can make the most out of real-time data and analytics tools, and we speak to Medidata about its efforts to integrate wearable health trackers such as Fitbits into the drug development and clinical trial process to improve the collection of patient data.
Read the issue for free on your iPad through our app, or if you’re on a desktop computer you can also read it in our web viewer.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn this issue
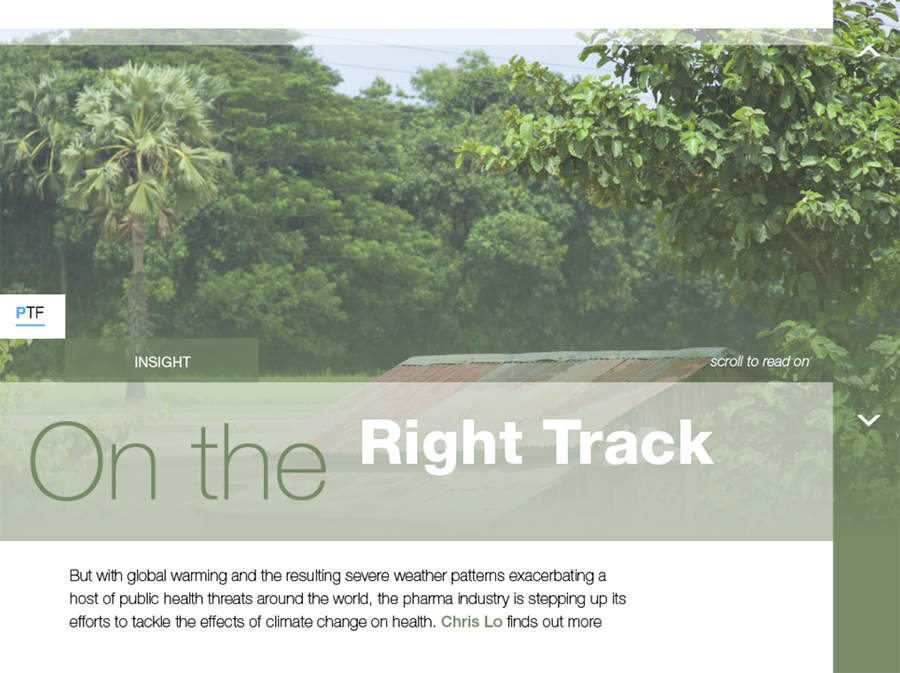
On the Right Track
With global warming and the resulting severe weather patterns exacerbating a host of public health threats around the world, the pharma industry is stepping up its efforts to tackle the effects of climate change on health. Chris Lo finds out more.
Read the article.
The Road to Russia
Russia is emerging as a popular study location thanks to high recruitment rates and low costs, but challenges remain. Elly Earls explores the country’s future as a market for clinical trials.
Read the article.
The Power of the Microbiome
It’s known that bacteria affect the way we interact with medicines, but the underlying mechanisms and their potential for drug development are only just being explored. Abi Millar speaks to the biotechnology company at the forefront of this research.
Read the article.
Joining Forces
Pharma companies from around the globe have declared their commitment to fighting antimicrobial resistance. Mirco Junker, PhD, GlobalData’s analyst for infectious diseases, reports on the need for a concerted effort and incentives for innovative R&D.
Read the article.
Analytics: the Key to Innovation?
Analytics and on-demand data insight are high on the agenda in many industries, but the pharma industry has yet to make the most of information management strategies. Elvis Pacelat, vice president for compliance solutions at AMPLEXOR Life Sciences, explains how analytics can become a source of innovation and competitive advantage.
Read the article.
mHealth: Shaking up the Template
Activity trackers are growing ever more popular with consumers, but could they also find an application in clinical research? Abi Millar finds out how wearables could be integrated into the drug development process.
Read the article.
Next issue preview
Israel’s pharmaceutical and biotechnology market has a reputation for efficiency, high R&D spending and an impressive international reach, with world-class export numbers and a growing market value. However, could weak intellectual property laws and long drug approval times prove to be a disincentive to investment from multinational firms? We profile this market and get an outlook for the years ahead.
The idea of delivering insulin via inhaler has been around for some time and has clear benefits for patients, but progress has been slow. We investigate where the problems lie. We also take a look at the FDA’s new draft guidelines aimed at modernising manufacturing practices in the US, explore which clinical factors in the field of obstetrics still need to be improved, and find out why a new class of sound wave discovered recently could have profound implications on stem cell research.
Digital magazine FAQ
You can read Pharma Technology Focus for free on the iPad. Download our app from the App Store to read the latest issue and browse the back issues in our archive. Sign up for a in the app and never miss a new issue.
You can also continue to read the desktop version for free on our web viewer. (Browser compatibility: The web viewer works in the latest two version of Chrome, Firefox and Safari, as well as in Internet Explorer 9 and 10. Some features may not be compatible with older browser versions.)



