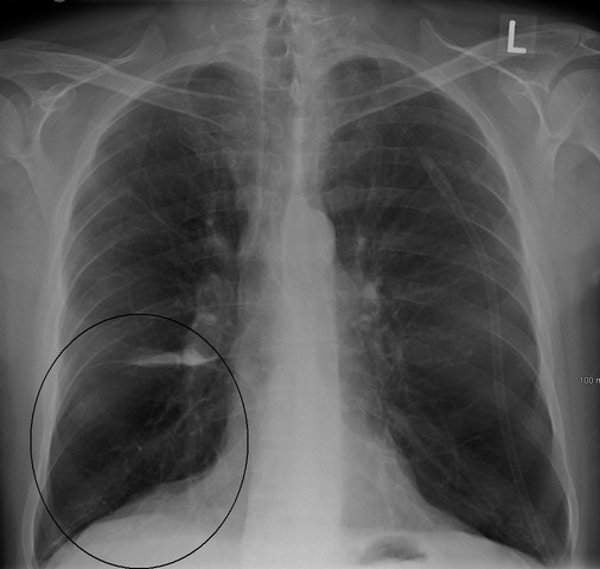
GSK and Theravance report initial results of SUMMIT COPD survival study

GlaxoSmithKline (GSK) and Theravance reported the initial results for Relvar / Breo Ellipta 100mcg / 25mcg (fluticasone furoate ‘FF’ / vilanterol) as part of the study to understand mortality and morbidity in chronic obstructive pulmonary disease (COPD) (SUMMIT).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Participants in the study included around 16,485 patients suffering from COPD with moderate airflow limitation and increased risk of cardiovascular disease (CVD).
The risk of dying on FF/VI 100mcg / 25mcg was found to be 12.2% lower than on placebo during the primary endpoint of the trial.
GSK global respiratory franchise senior vice-president and head Eric Dube said: "SUMMIT is an important study as this is the first time that survival has been studied in this under-researched co-morbid patient population."
AstraZeneca reports positive Phase III results of CAZ-AVI in patients with cUTI
AstraZeneca reported positive topline results from RECAPTURE 1 and RECAPTURE 2, the pivotal Phase III trials evaluating the antibiotic, CAZ-AVI, as a treatment for hospitalised adults with complicated urinary tract infections (cUTI), including pyelonephritis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataCAZ-AVI is a combination of cephalosporin (ceftazidime), an established treatment for serious bacterial infections, and avibactam, a next-generation non-beta lactam beta-lactamase inhibitor.
It is being developed to treat a broad range of gram-negative bacterial infections which are becoming increasingly resistant to antibiotics.
Merck and Syndax begin dosing in Phase Ib / II trial of entinostat and Keytruda

Merck and Syndax Pharmaceuticals started dosing first patients in the Phase Ib / II clinical trial of entinostat in combination with Keytruda in patients with non-small cell lung cancer (NSCLC) or melanoma.
Entinostat is Syndax’s oral, small molecule that targets immune regulatory cells, while Keytruda (pembrolizumab) is Merck’s anti-programmed cell death protein 1 (anti-PD-1) antibody.
The trial, designated ENCORE 601 by Syndax and KEYNOTE 142 by Merck, is designed to evaluate the safety, tolerability and efficacy of the immuno-oncology combination in these patients.
Boehringer and Lilly’s diabetes drug Jardiance shows reduction in cardiovascular risk
Boehringer Ingelheim Pharmaceuticals and Eli Lilly and Company presented the results of the EMPA-REG OUTCOME trial of Jardiance (empagliflozin) in type 2 diabetes patients at high-risk for cardiovascular (CV) events.
Jardiance is a once-daily pill taken in the morning, used along with diet and exercise, to lower blood sugar (A1C) in adults with type 2 diabetes.
The drug showed a significant reduction in both CV risk and death in the long-term, multicentre, randomised, double-blind, placebo-controlled trial.
Bristol-Myers reports positive results from two opdivo trials in NSCLC patients
Bristol-Myers Squibb (BMS) reported positive results from two pivotal trials CheckMate -017 and -063 evaluating opdivo in previously treated squamous (SQ) non-small cell lung cancer (NSCLC).
The longer term survival and safety data showed sustained survival benefit across both the trials.
Opdivo showed an estimated 18 month overall survival (OS) rate of 27% in CheckMate -063 to 28% in CheckMate -017, while survival benefit was independent of PD-L1 expression.
Galectin initiates Phase IIa pilot trial of GR-MD-02 to treat psoriasis
US-based Galectin Therapeutics started a ten-patient Phase IIa pilot trial with GR-MD-02, a complex carbohydrate drug, to treat moderate-to-severe plaque psoriasis.
The company is a developer of therapeutics that target galectin proteins to treat fibrosis and cancer.
The genesis of the pilot trial is the apparent remission of a patient with severe psoriasis who participated in the company’s Phase I trial group 4mg/kg of GR-MD-02 to treat non-alcoholic steatohepatitis (NASH).
Corbus enrols first patient in Phase II trial of Resunab to treat cystic fibrosis in adults
US-based Corbus Pharmaceuticals Holdings started patient enrolment in the Phase II clinical trial of its investigational new drug Resunab to treat adult patients with cystic fibrosis (CF).
Resunab is a new oral drug targeting the resolution of inflammation and fibrosis associated with disease progression in CF across all cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations.
It is a new synthetic oral endocannabinoid-mimetic drug that preferentially binds to the CB2 receptor expressed on activated immune cells and fibroblasts.
Revance begins Phase II trial of botulinum toxin type A for injection to treat cervical dystonia

US-based biopharmaceutical firm Revance Therapeutics started dosing in a Phase II dose-escalating clinical trial of an investigational drug product candidate, RT002, to treat cervical dystonia, a neurological muscle movement disorder.
The company is focused on developing botulinum toxin products for use in aesthetic and therapeutic indications.
The trial is designed to evaluate the safety, preliminary efficacy and duration of effect of a single treatment of RT002 (RTT150 (Botulinum Toxin Type A) for injection) in patients with moderate-to-severe isolated cervical dystonia symptoms of the neck.
Revance president and chief executive officer Dan Browne said: "Cervical dystonia is a debilitating condition characterised by involuntary muscle contractions in the neck. This painful malady is the first therapeutic indication we’re pursuing for our unique injectable neurotoxin.



