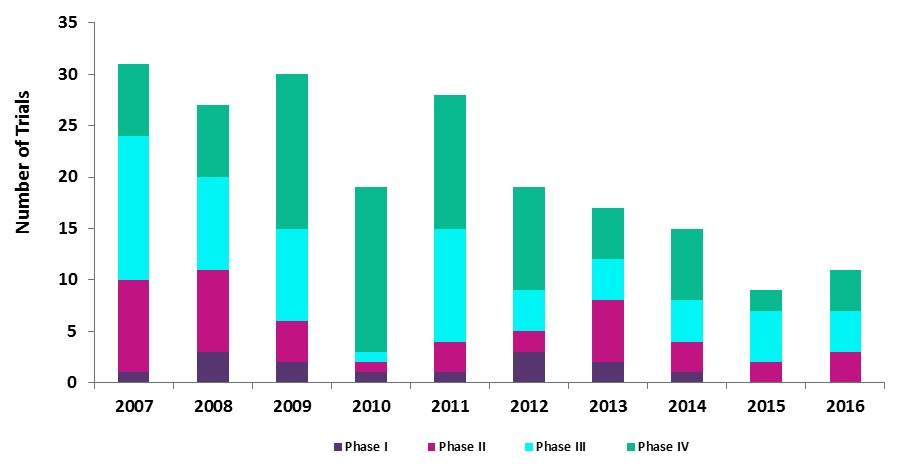
Analysis conducted by GlobalData has found that the rate initiation of trials studying Human Papillomavirus Vaccines between 2007 and 2016 has seen a gradual decrease from a high of 31 trials launched in 2007 to 11 trials instigated in 2016, a reduction of 15% over the ten-year period.
As shown in Figure 1, the dominating phases over this period were both Phase III and Phase IV trials. Despite the over-arching contraction, both of these phases have maintained an average of approximately 32% in their yearly distribution.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
While all phases experienced a downturn within the first four years, the initiation of Phase IV trials increased by almost 44% from seven in 2007 to 16 in 2010. However, this trend underwent a reversal from 2011 onwards. In 2015, Phase IV trials had one of the largest slumps of all phases, with a 71% fall in the study initiation rate.
Figure 1 displays the number of Biosimilar Clinical Trials by Phase Type 2007-2016.

Source: GlobalData, Pharma Intelligence Center, Clinical Trials Database [Accessed July 27, 2017]

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThese findings support a review that recently discovered the incidence of infections caused by HPV has been cut by almost 90%. More than 187 million doses of HPV vaccine have been administered in more than 130 countries, including the US and Australia.
This means that the incidence of cancers caused by the HPV, approximately 70% of all cervical cancer, is also on the fall.





