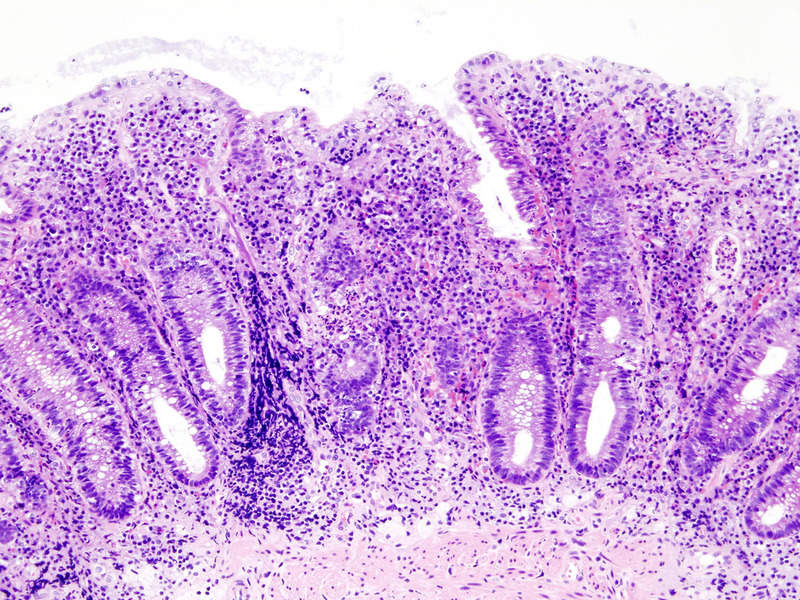
Abivax has started patient enrolment in a Phase IIa clinical trial (ABX464-101) of its product candidate ABX464 for the treatment of moderate-to-severe active ulcerative colitis.
ABX464 is an investigational small molecule being developed to impart strong anti-inflammatory activity.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The Phase IIa trial is designed to assess the safety and efficacy of a 50mg once-daily dose of ABX464 over eight weeks in 30 patients who failed or are intolerant to immunomodulators, anti-TNFα, vedolizumab and/or corticosteroids.
Abivax is set to conduct the trial at 18 sites across France, Germany, Belgium, Poland, Czech Republic, Spain, Hungary and Austria, and has already received regulatory and ethics committee authorisations in France, Belgium and Hungary.
The University Hospitals Leuven in Belgium has enrolled the first subject.
Abivax CEO Hartmut Ehrlich said: “Exciting preclinical and in-vitro human data showing ABX464’s efficacy in inflammatory bowel disease models led us to undertake this trial in patients with ulcerative colitis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“For example, ABX464 raised IL-22 and miR124 levels in in-vitro human immune cells by fifty and tenfold respectively, indicating that ABX464 has powerful anti-inflammatory properties and is potentially synergistic with some of the established therapies for ulcerative colitis.”
The primary objective of the trial is safety and tolerability, while efficacy objectives include clinical and biologic remission and mucosal healing.
With top-line results expected to be available in the second half of next year, the firm has submitted a follow-up protocol to enable subjects to continue treatment in a 12-month open-label follow-up study.





