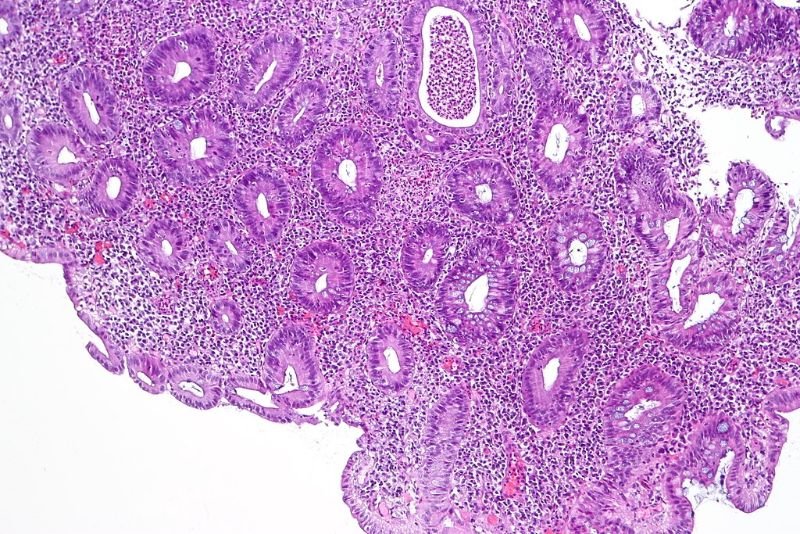
The US Food and Drug Administration (FDA) has approved Abivax’s investigational new drug (IND) application for ABX464 to treat moderate-to-severe ulcerative colitis (UC).
The approval enables the company to conduct clinical trials, including the ongoing Phase IIb ABX464-103 study, in the country. Patient enrolment for ABX464-103 in the US is expected to start in the second quarter of this year.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
ABX464 is an oral candidate that acts via upregulation of microRNA-124. The drug candidate employs anti-inflammatory effects by attaching to the cap-binding complex (CBC) at the 5′ end of RNA molecules.
Binding to the CBC is said to boost the complex’s biological functions in the biogenesis of cellular RNA. Abivax added that ABX464 does not affect the splicing of cellular genes.
The Phase IIb ABX464-103 trial is currently assessing the drug candidate in 232 participants across 15 European countries and Canada.
In November last year, the French regulatory agency granted approval for Abivax to perform the study in moderate-to-severe active UC patients in the country. ABX464-103 is designed to compare a 25mg/day, 50mg/day and 100mg/day once-daily dose of the drug to placebo.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAbivax CEO Hartmut Ehrlich said: “We are very excited about the green light from the FDA, which is a very important milestone in Abivax’s global development strategy for our lead compound, ABX464.
“By expanding the ongoing clinical Phase IIb study of ABX464 to the US, Abivax is aiming to make this new potential treatment option available to a substantial number of UC patients in need of new therapeutic solutions.”
According to results from a Phase IIa open-label maintenance study, ABX464 led to clinical remission in 75% of moderate-to-severe active UC patients who experienced an inadequate response to immunomodulators, vedolizumab and/or corticosteroids.
The drug candidate was found to be safe and well-tolerated in all studies, with no serious adverse reactions. The most common adverse events were headache, abdominal pain and diarrhoea.





