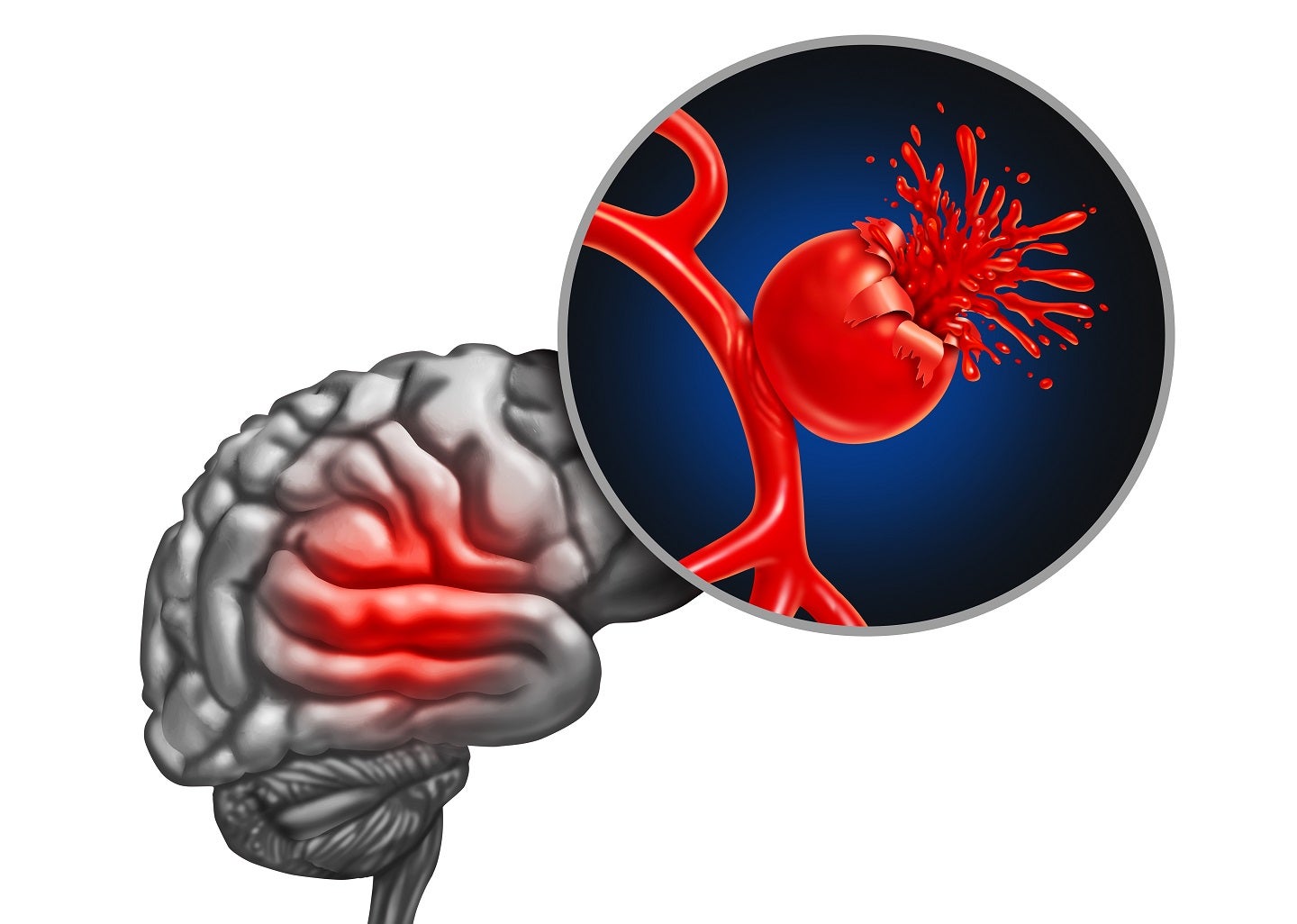
Acasti Pharma has submitted the full pivotal Phase III safety study protocol with all supporting documentation of GTX-104, a novel formulation of nimodipine for treating aneurysmal subarachnoid haemorrhage (aSAH), to the US Food and Drug Administration (FDA).
aSAH is bleeding that occurs over the brain surface in the subarachnoid space between the brain and the skull.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The company is also implementing a strategic realignment plan to maximise shareholder value until the second quarter of 2025.
Acasti Pharma CEO Prashant Kohli said: “We expect this pivotal Phase III Safety Study will be the final clinical step required to seek FDA approval under the 505(b)(2) regulatory pathway.
“As a result of the positive progress made with GTX-104, and following a strategic review, we felt it was critical to move swiftly and boldly to implement a plan that we believe will benefit Acasti’s shareholders by prioritising resources to this high-value asset.”
Pending FDA’s final feedback and approval, the first patient is expected to be dosed in the fourth quarter of this year.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn addition, Acasti is appointing new team members who have deep subject matter knowledge and direct, hands-on clinical trial experience in aSAH.
With the strategic realignment, Acasti is discontinuing its operations in Canada enabling a new management team to rebuild a leaner organisation in the US.
Kohli added: “The new Acasti is a highly motivated and energised organisation that is flatter, agile, and strategically closer to our addressable market opportunity in the US.”





