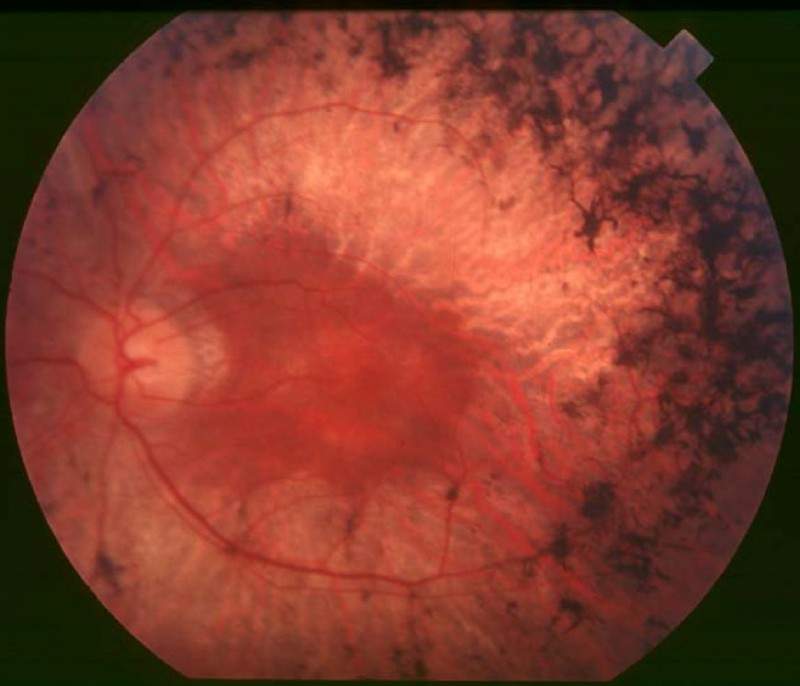
Applied Genetic Technologies (AGTC) has enrolled the first patient of the second cohort in a Phase l/ll clinical trial to examine the safety and efficacy of an unnamed investigational AAV-based gene therapy for the treatment of X-linked retinitis pigmentosa (XLRP).
The open-label, dose-escalation trial is being conducted as part of a collaboration between AGTC and Biogen.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Under the collaboration, AGTC will receive a milestone payment of $10m from Biogen.
AGTC president and CEO Sue Washer said: “We are pleased to announce this important milestone under our collaboration with Biogen and remain on track to complete the dose-escalation portion of the trial in the first quarter of 2019.”
The Phase l/ll trial aims to evaluate the safety and efficacy of subretinal administration of the AAV-based gene therapy in patients diagnosed with XLRP caused by mutations in the retinitis pigmentosa GTPase regulator (RPGR) gene.
The trial’s primary focus is to assess the safety of the vector and subretinal delivery procedure by analysing focal (ocular) and systemic treatment-emergent adverse events.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt is also expected to measure biologic activity by assessing changes in visual function, retinal structure and quality of life.
Topline six-month data from the trial is expected to be released by the end of this year, with primary analysis of the full 12 month active trial data planned to be provided after six months.
XLRP is an inherited condition that gradually causes loss of vision in boys and young men.
The disease is characterised by night blindness in early childhood and progressive constriction of the visual field.





