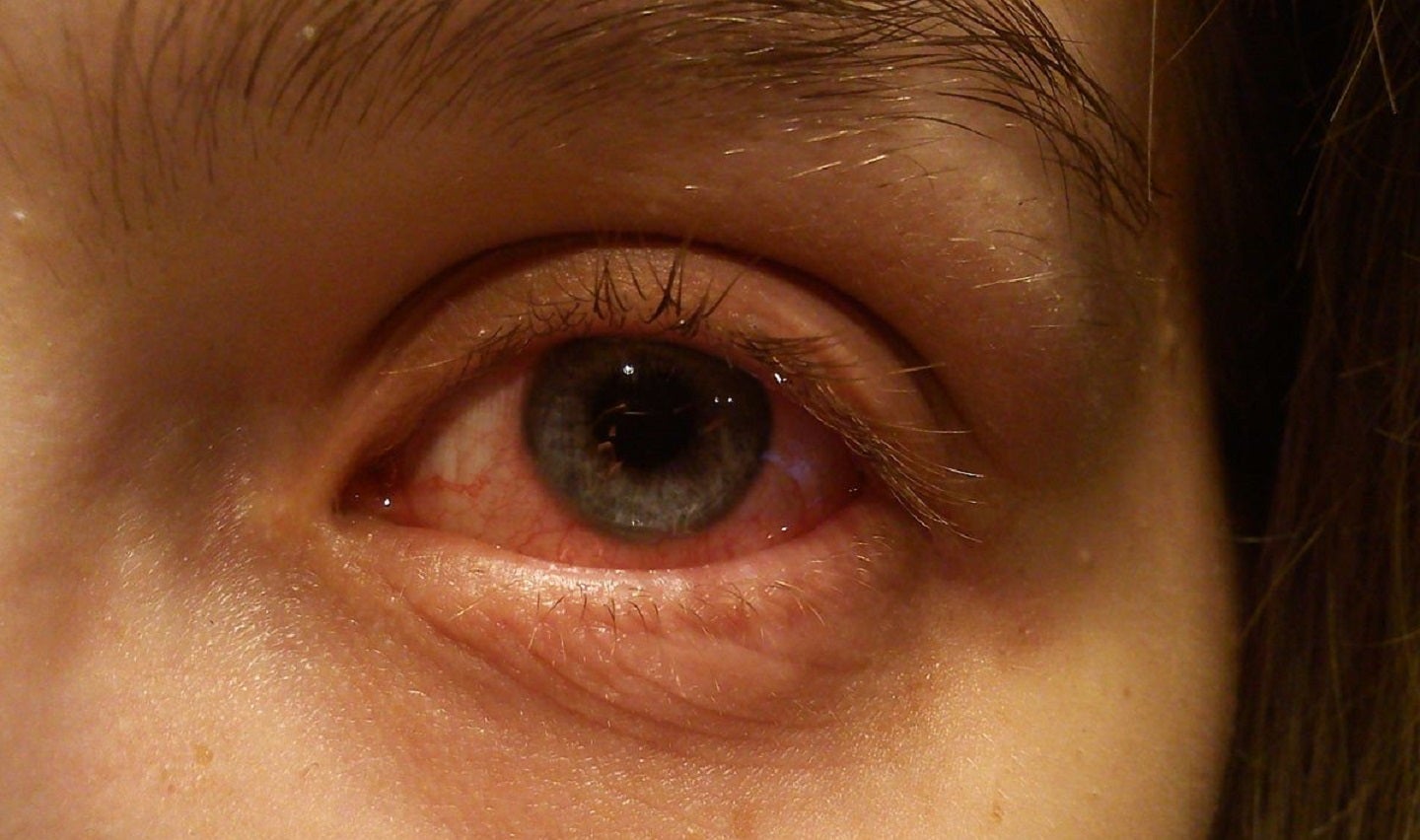
Aldeyra Therapeutics has concluded enrolment in the Phase III INVIGORATE-2 trial of its topical ocular reproxalap for the treatment of patients with allergic conjunctivitis.
Said to be the most common inflammatory disease, allergic conjunctivitis affects the front of the eye.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
A total of 131 patients with seasonal allergic conjunctivitis were enroled in the double-masked, crossover, randomised, vehicle-controlled trial.
Subjects were exposed with real-world pollen in an allergen chamber and evaluated for 3.5 hours.
Patient-reported ocular itching is measured as the primary endpoint of the trial.
Aldeyra president and CEO Todd Brady said: “Today, millions of allergic conjunctivitis patients rely on therapies that may not provide sufficient relief or cannot be used chronically due to serious side effects.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Reproxalap, which has demonstrated rapid and durable improvement in the symptoms and signs of allergic conjunctivitis across a number of clinical trials, could signify the first new therapeutic mechanism of action in decades for patients suffering from this persistently disturbing condition.”
Top-line results from INVIGORATE-2 are expected to be announced in the first half of this year.
Reproxalap, a small-molecule reactive aldehyde species (RASP) modulator, has been investigated in over 2,300 subjects and no clinically significant safety concerns were observed.
INVIGORATE-2’s protocol is substantially identical to that of the Phase III INVIGORATE and Phase II clinical trials. Both the trials achieved the ocular itching endpoint (P<0.001).





