Alnylam Pharmaceuticals has reported that the Phase III ENVISION clinical trial of givosiran has met its primary endpoint with reduction in the annualised rate of composite porphyria attacks compared to placebo in patients with acute hepatic porphyria.
Top-line results also showed that the trial met five of nine secondary endpoints, demonstrating statistically significant results.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
However, the trial failed to meet the secondary endpoints of daily worst pain, daily worst fatigue, daily worst nausea and the physical health component of a quality-of-life survey.
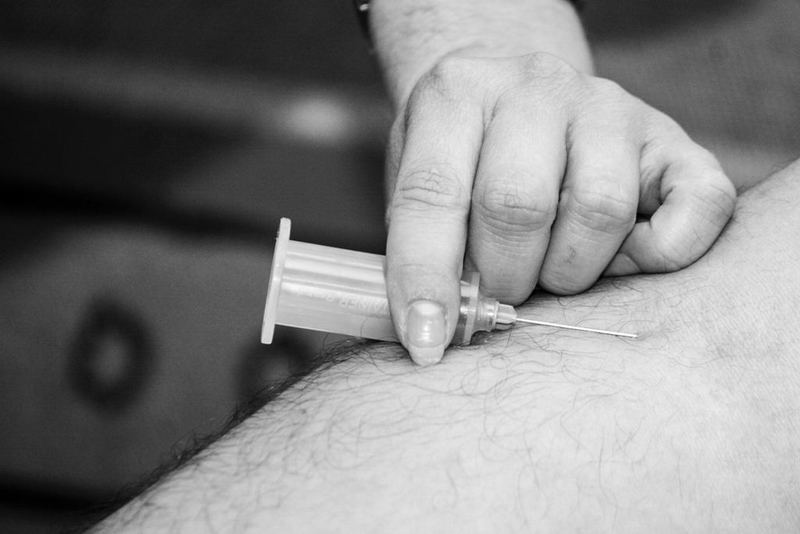
Givosiran is an investigational, subcutaneous RNAi therapeutic designed to target aminolevulinic acid synthase 1 (ALAS1) and lead to a decrease in neurotoxic heme intermediates.
The randomised, double-blind, placebo-controlled ENVISION trial was conducted to evaluate 2.5mg/kg monthly givosiran in 94 acute hepatic porphyria patients at 36 sites across 18 countries.
The primary endpoint of a decrease in the annualised rate of composite porphyria attacks was defined as the proportion of acute intermittent porphyria patients requiring hospitalisation, urgent healthcare visit or hemin administration over six months.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataGivosiran is said to have demonstrated an encouraging safety and tolerability profile during the trial.
Adverse events (AEs) were experienced by 89.6% of the patients treated with givosiran, compared to 80.4% of patients on placebo. Almost 20.8% of patients in the givosiran group reported serious adverse events (SAEs) as against 8.7% of placebo patients.
Alnylam Pharmaceuticals CEO John Maraganore said: “Notably, givosiran is the first GalNAc-conjugate siRNA to achieve positive Phase III results, and the second example of Alnylam’s R&D strategy bearing fruit due to our focus on genetically validated targets for the advancement of potential high-impact medicines.
“Assuming favourable regulatory review, we very much look forward to adding givosiran as the second product in our global commercialisation efforts.”
Based on results from the Phase III trial, the company intends to complete its new drug application (NDA) and marketing authorisation application (MAA) submission in mid-2019.





