Arrowhead Pharmaceuticals has initiated a potentially pivotal Phase II/III clinical trial of ARO-AAT, its second generation subcutaneously administered RNA interference (RNAi) therapeutic.
As part of the initiation, the company dosed the first patient in the SEQUOIA (AROAAT2001) trial.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
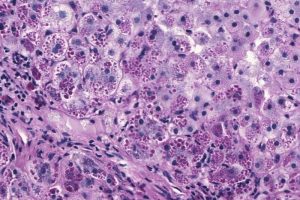
Arrowhead is developing the RNAi therapeutic as a treatment for a rare genetic liver disease associated with alpha-1 antitrypsin deficiency (AATD).
Arrowhead Pharmaceuticals chief operating officer and R&D head Bruce Given said: “ARO-AAT is the first RNAi therapeutic derived from our proprietary Targeted RNAi Molecule, or TRiM, platform to reach a potentially pivotal study.
“This is a significant milestone for Arrowhead and, more importantly, it represents potential hope for patients living with alpha-1 liver disease, who currently have no available treatment options other than liver transplant.”
The placebo-controlled, adaptive design Phase II/III SEQUOIA (NCT03945292) trial will evaluate the safety, efficacy, and tolerability of the drug candidate ARO-AAT administered subcutaneously to patients with AATD associated liver disease.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataA total of 120 patients will be given at least nine doses, or approximately two years worth of treatment, with ARO-AAT or placebo.
Doses will then be administered on day one, 29, and roughly every 12 weeks.
Part A of the study will be four-arm and placebo-controlled and will feed seamlessly into a two-arm placebo-controlled Part B component.
The primary objective for Part A is to select a single dose level for use in Part B of the study, while the primary objective for Part B is to evaluate the efficacy of ARO-AAT.
In September last year, Arrowhead completed dosing patients in a Phase I clinical trial (AROAAT1001) of ARO-AAT for the treatment of a liver disease caused by AATD.





