Arthrosi Therapeutics, a biotechnology company specialising in gout treatment, has successfully secured $75m in Series D financing.
Led by Guangrun Health Industry, the financing round was supported by several investors, including ApicHope Pharmaceuticals subsidiary Reichstein Biotech.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
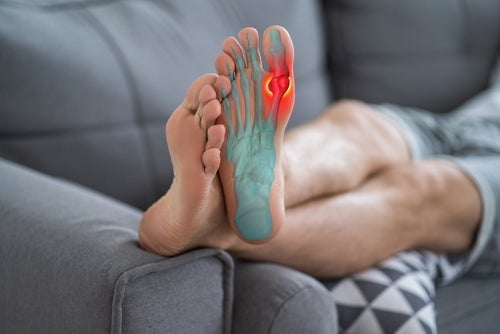
Founded in 2018, US-based Arthosi will use the financing to further support the development of AR882. The drug candidate is a selective next-generation URAT1 inhibitor delivered in a once-daily immediate-release oral capsule, which proposes to reduce serum uric acid (sUA) levels, flares and tophi, a condition where uric acid crystallises in joints causing pain and swelling.
URAT1 mediates the re-absorption of uric acid in the kidneys, thereby playing a key role in uric acid homeostasis. Circulating uric acid is thought to protect against bone mineral density loss and oxidative damage. However, excess sUA results in gout, hypertension, and cardiovascular disease.
Phase 2b trials (NCT05253833) for AR882 have demonstrated safety and efficacy, with high response rates, achieving the minimum sUA target of below 6mg/dL and achieving targets below 5mg/dL or 4mg/dL for faster flare reduction and dissolution of crystal deposition and tophi.
Arthrosi CEO Litain Yeh said: “I am not aware of any other molecule that has shown such promising results.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We continue to be extremely positive about how AR882 can address the significant unmet need in the gout space. In the US alone, there are nine million people who suffer from limited treatment options. The Series D financing and continued partnership with ApicHope will accelerate the development of AR882 and other groundbreaking drugs in Western countries and in Asia.”
In June 2023, a competitor URAT1 inhibitor, Urica, reported Phase I trial data of dotinurad. The trial found the drug to be safe and well-tolerated. Phase 1b clinical trials for that drug are expected to begin in 2024.



