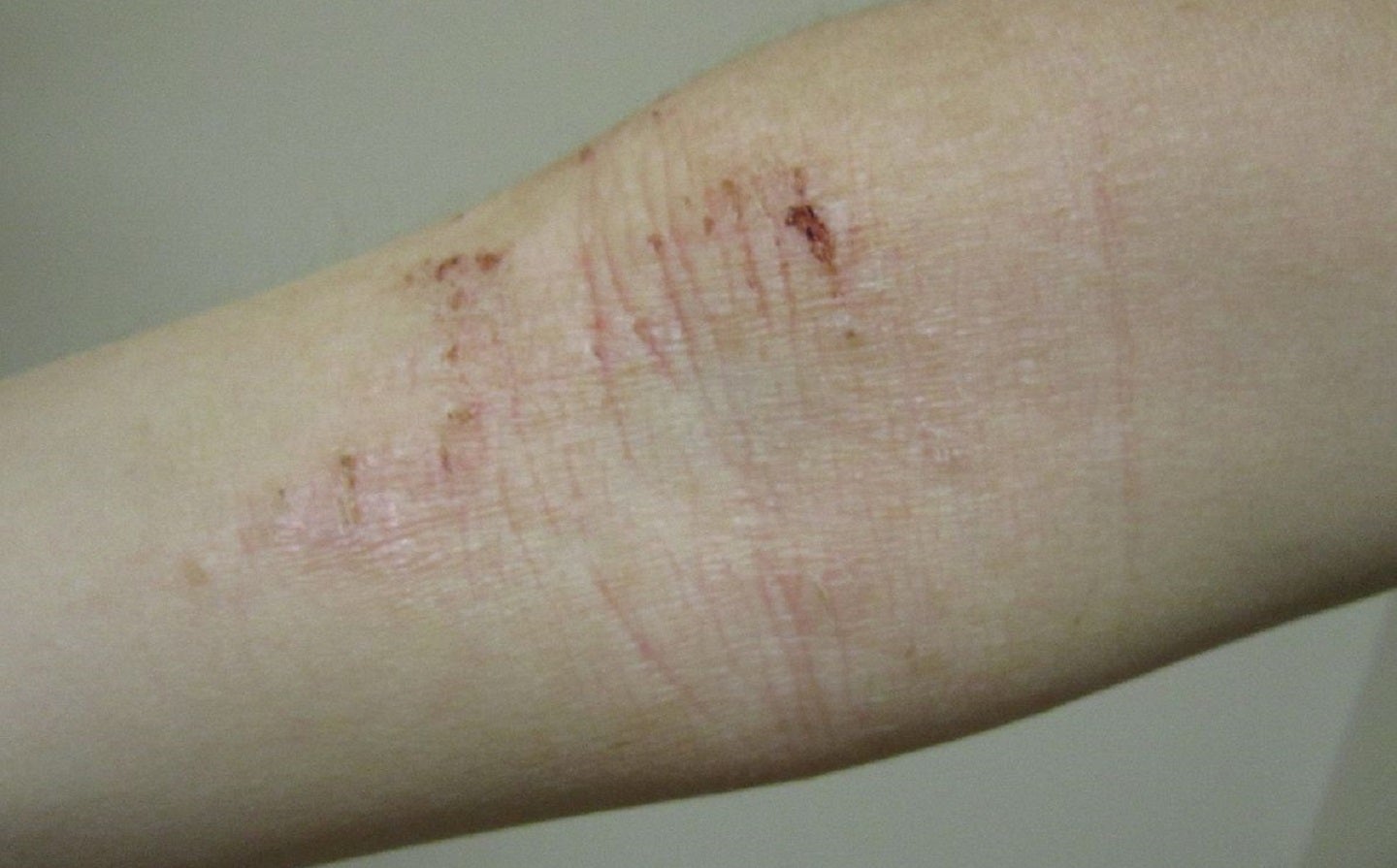
Aslan Pharmaceuticals has enrolled the final subject in the TREK-AD Phase IIb clinical trial of its monoclonal antibody eblasakimab to treat moderate-to-severe atopic dermatitis (AD).
The dose-ranging, randomised, placebo-controlled, double-blind trial of eblasakimab in adult subjects was initiated last year.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It has been designed for assessing eblasakimab’s safety and efficacy in biologic naïve AD patients during a 16-week treatment period, and a safety follow-up period of 12 weeks.
The trial has enrolled subjects from over 80 centres across Europe, North America, and the Asia Pacific.
The percentage change in Eczema Area Severity Index (EASI) score from baseline to week 16 is the trial’s primary efficacy endpoint.
Secondary efficacy endpoints include the proportion of patients with a 75% or more decline in EASI (EASI-75) and the proportion of those achieving a validated Investigator Global Assessment (vIGA) score of zero (clear) or one (almost clear).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe proportion of subjects achieving EASI-50 (a 50% or greater reduction) and EASI-90 (a 90% or greater reduction), and changes in peak pruritus scores are also included as secondary efficacy endpoints.
Topline data from the trial is expected in early July this year.
Aslan Pharmaceuticals CMO Dr Alex Kaoukhov said: “The findings will provide greater clarity on eblasakimab’s differentiation and, combined with data from the ongoing TREK-DX study, will support its potential as a biologic of first-choice for AD patients.
“We are grateful to all patients, physicians, and trial investigators involved in the study. Their involvement is testament to the need that exists for new therapies to treat AD.”
In the Phase II TREK-DX trial, the company is also studying the antibody in dupilumab experienced, moderate-to-severe AD patients. Data from this trial is expected in the first quarter of next year.





