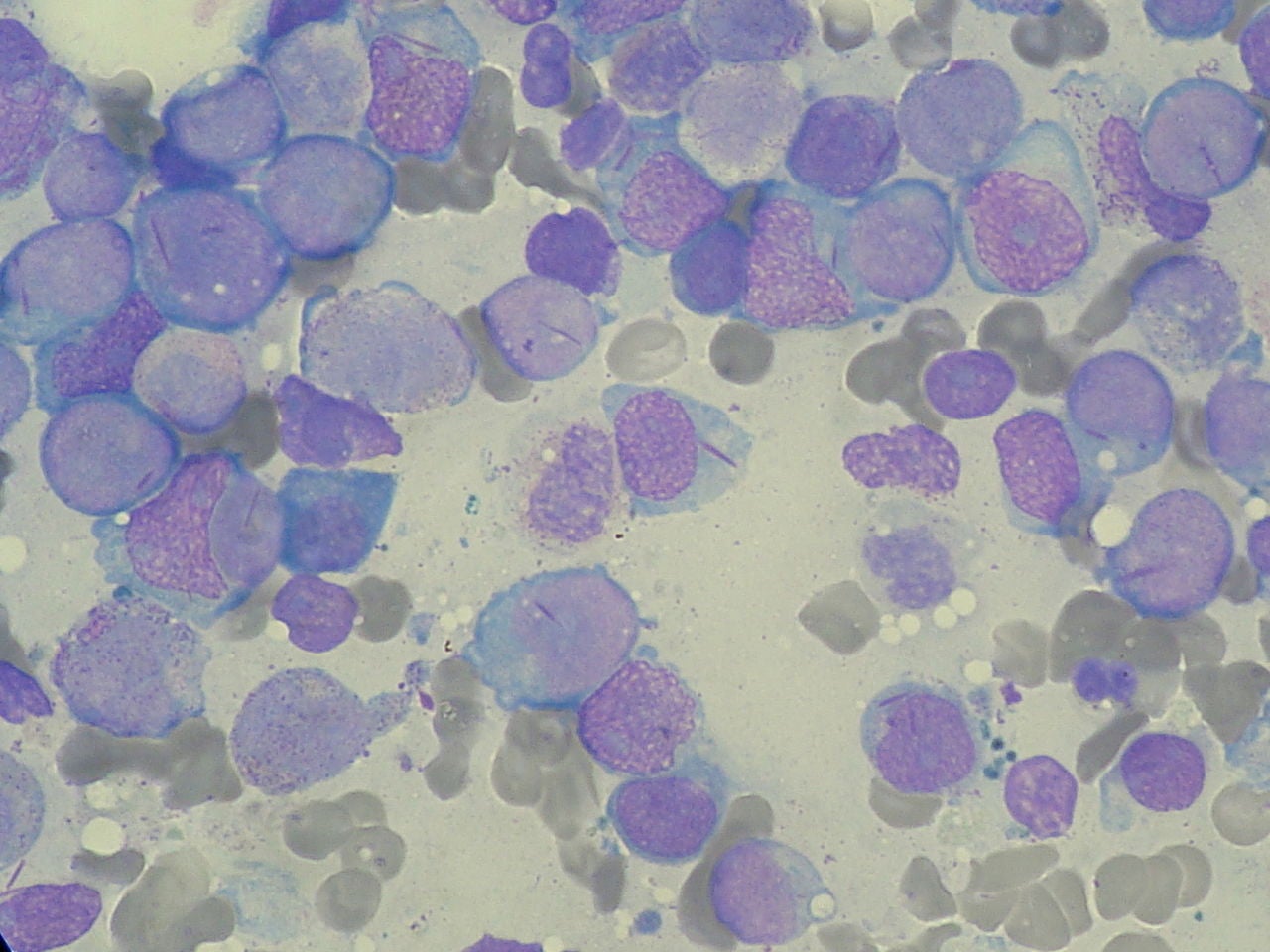
Astex Pharmaceuticals has reported that Phase III trials of guadecitabine (sSGI-110) failed to meet the primary endpoint of improving overall survival (OS) in leukaemia patients.
ASTRAL-2 and ASTRAL-3 trials evaluated the safety and efficacy of guadecitabine for patients with acute myeloid leukaemia (AML) and myelodysplastic syndromes or chronic myelomonocytic leukaemia (MDS / CMML), respectively.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
In the ASTRAL-2 trial, the hypomethylating agent guadecitabine was evaluated in adults with refractory / relapsed AML who were earlier treated with initial induction therapy using an intensive chemotherapy regimen.
The trial randomised 302 participants from 98 sites in 15 countries globally. Patients received subcutaneous guadecitabine in 28-day cycles or physicians’ choice of drugs or supportive care.
It assessed secondary endpoints including event-free survival, long-term survival, number of days alive and out of the hospital, disease response, complete response rate and quality of life, among many others.
The ASTRAL-3 trial evaluated guadecitabine in adults with MDS or CMML treated earlier with a hypomethylating agent. It enrolled 417 participants from 91 sites in 14 countries across the globe.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn 28-day cycles, patients received subcutaneous guadecitabine or physicians’ choice of drugs / chemotherapy or supportive care.
Multiple secondary endpoints including transfusion independence, marrow complete response rate, survival rate, leukaemia-free survival and others were evaluated in this study.
Astex president and chief medical officer Mohammad Azab said: “The ASTRAL series of studies was designed to deliver a new therapeutic option to patients with AML or MDS / CMML, and although guadecitabine is an active drug, the studies failed to demonstrate a statistically superior survival outcome compared to current therapeutic alternatives.”
“Guadecitabine was associated with improved outcomes in certain subgroups, but that needs to be validated by additional studies.”





