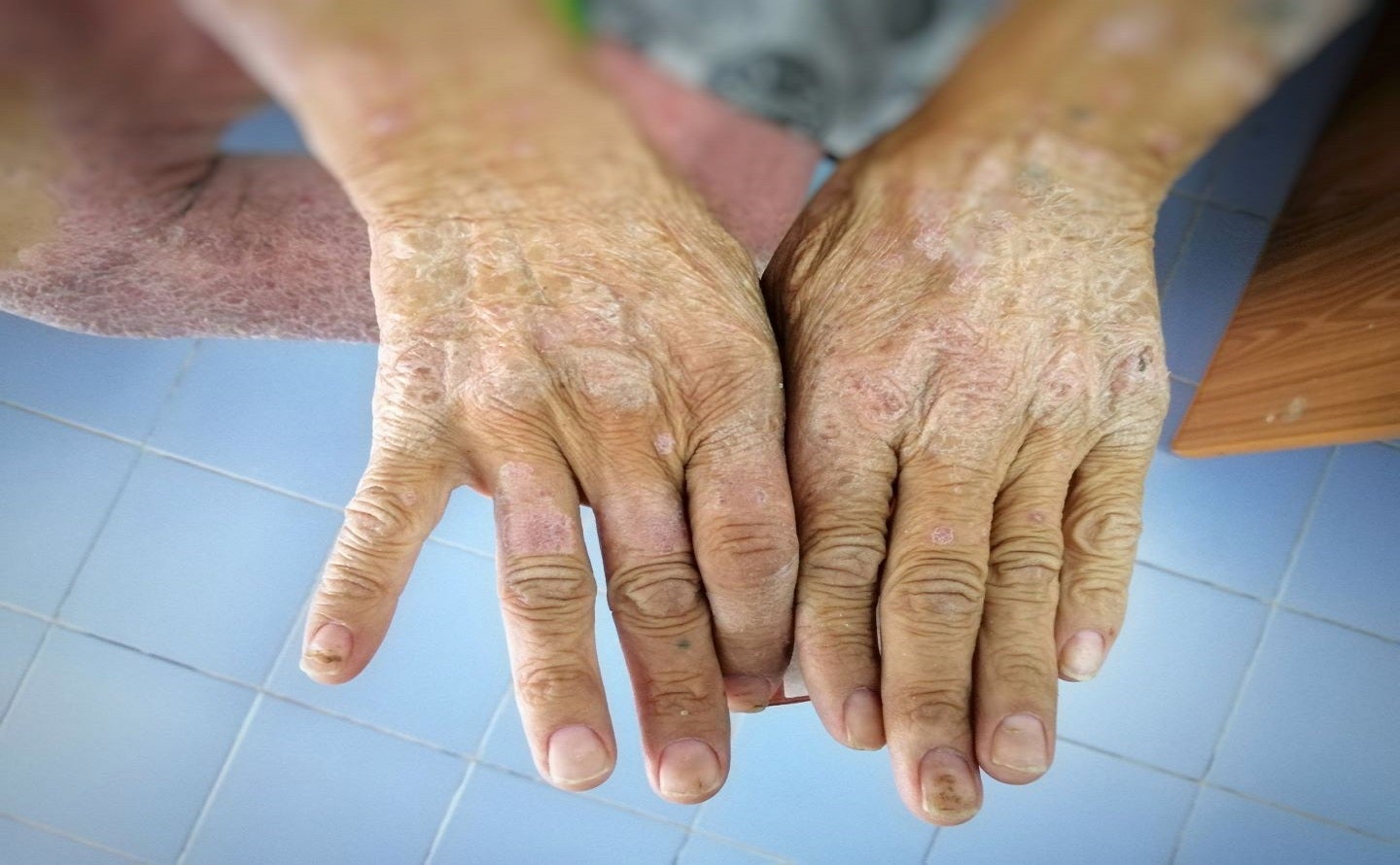
aTyr Pharma has dosed the first subject in the Phase II EFZO-CONNECT trial of efzofitimod to treat systemic sclerosis (SSc, or scleroderma)-related interstitial lung disease (ILD).
The proof-of-concept (PoC) trial is designed to assess the tolerability, efficacy, and safety of the therapeutic candidate against placebo in patients with this condition.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The double-blind, placebo-controlled 28-week study includes three parallel cohorts randomised into a 2:2:1 ratio to receive either 270mg or 450mg of efzofitimod or a placebo administered intravenously monthly for six doses.
In the trial, the company aims to enrol 25 patients at several centres in the US.
Assessing the efficacy of multiple intravenous efzofitimod doses on pulmonary, cutaneous and systemic manifestations in SSc-ILD patients is the study’s primary objective.
Safety and tolerability are the secondary objectives of the trial.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataEfzofitimod is a first-in-class biologic immunomodulator designed to selectively modulate activated myeloid cells through neuropilin-2 (NRP2) for resolving inflammation without immune suppression and preventing fibrosis progression.
The company respectively received the fast track and orphan drug designations from the US Food and Drug Administration (FDA) and the EU for efzofitimod to treat SSc.
aTyr president and CEO Sanjay Shukla said: “Efzofitimod has been shown preclinically to reduce lung and skin fibrosis in models of SSc, and NRP2, efzofitimod’s binding partner, is expressed in the skin of SSc patients.
“We believe there is compelling rationale that efzofitimod has the potential to target the underlying disease pathology central to this form of ILD and positively impact lung function and improve outcomes in these patients.”
The chronic autoimmune disease Systemic sclerosis is characterised by inflammation and fibrosis of connective tissues throughout the body.





