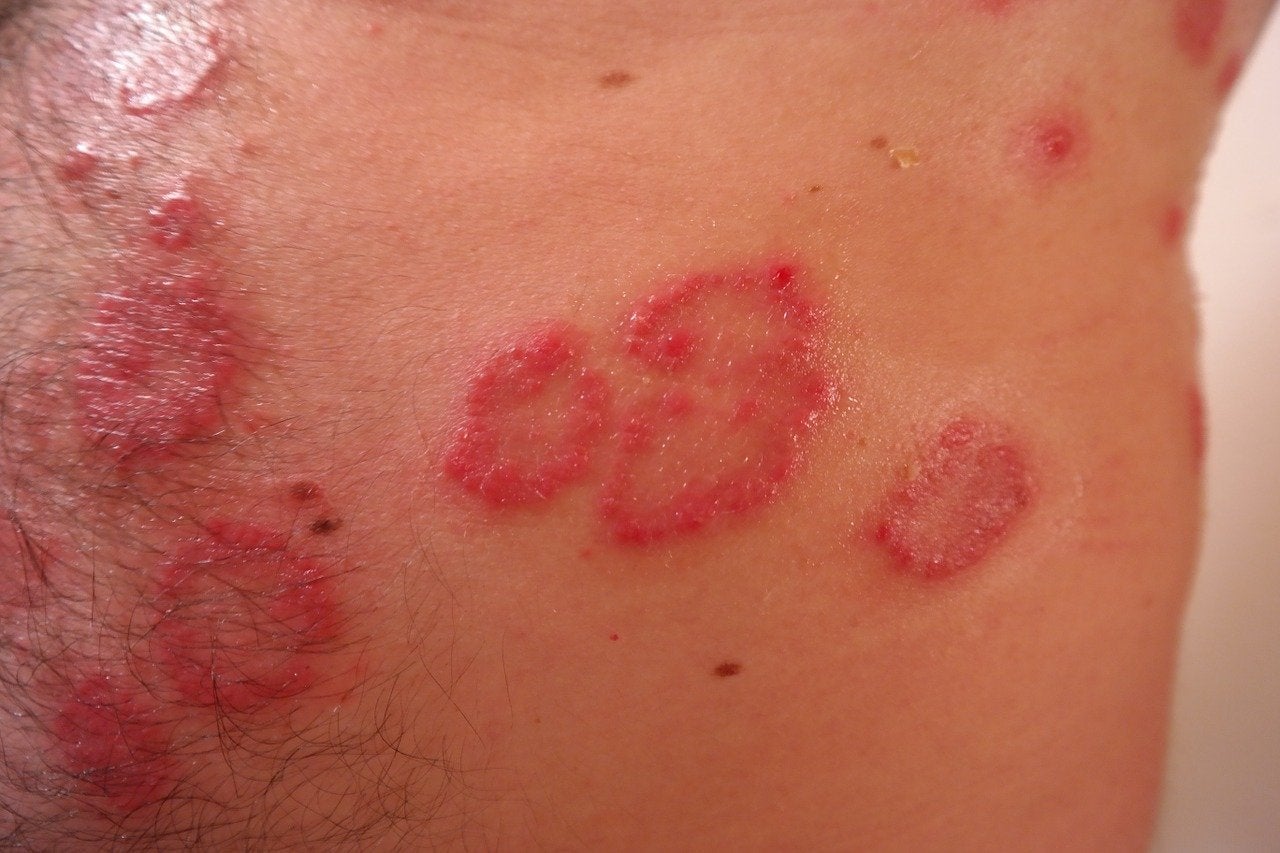
UK-based BenevolentAI has dosed the first patient in its first-in-human clinical trial of its novel multi-target drug, BEN-2293, for treating atopic dermatitis (AD).
Designed and developed using BenevolentAI’s scientific and technical expertise, BEN-2293 is a potent and selective small-molecule Pan-Trk antagonist formulated for topical delivery.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It has a pharmacokinetic profile designed for low systemic exposure to offer the optimal safety and efficacy profile in treating itch and inflammation linked to AD.
AD is characterised as a chronic relapsing inflammatory skin condition, which manifests as itchy, inflamed skin.
Estimates show that mild to moderate AD affects about 70% of all patients, with its occurrence rising globally.
BenevolentAI noted that the drug will be studied in adult subjects with mild to moderate AD in this initial trial.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAt present, the standard of care for the topical treatment of atopic dermatitis includes corticosteroids and calcineurin inhibitors which have usage limitations as well as adverse side effects.
There exists a huge unmet need for patients that are either non-responders or refractory to presently available treatments.
The company noted that BEN-2293 can potentially address both the inflammation and itch linked to AD in adults and children, indicating a comprehensive, new treatment available for a wider patient population.
BenevolentAI chief scientific officer Anne Phelan said: “The poor treatment options available for atopic dermatitis patients make the disease difficult to endure.
“New ways to treat this disease are desperately needed, and we believe our molecule has strong potential to address this serious unmet need without the use of topical steroids.
“The BEN-2293 trial also marks an important first step in the development of our in-house drug pipeline, and we are excited to be progressing this candidate into the clinic.”
The company has active R&D drug programmes in disease areas including ALS, ulcerative colitis and Oncology and research and commercial partnerships with AstraZeneca and Novartis.





