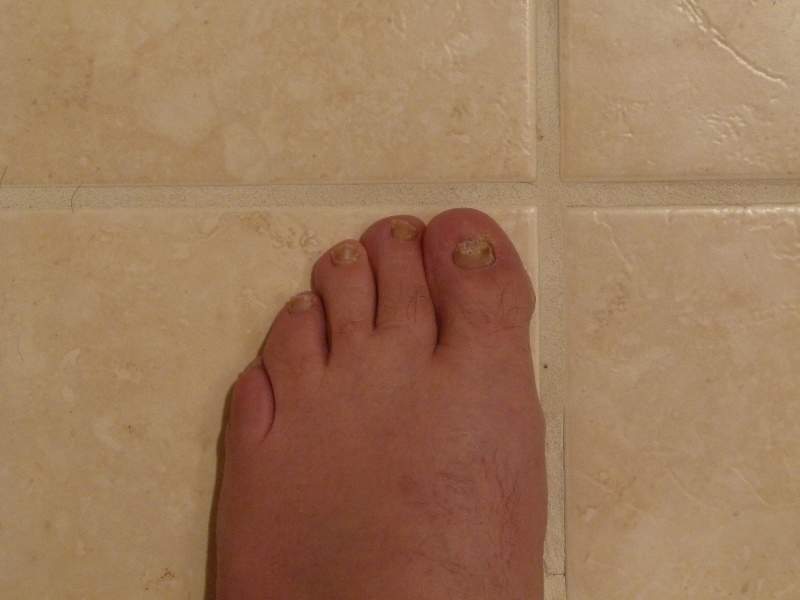
Blueberry Therapeutics has reported positive results from the Phase I/II trial evaluating the pharmacokinetic, safety and local tolerability of BB2603 for the treatment of dermatological disorders.
The single-centre, randomised trial met all of its primary and secondary objectives, which included systemic pharmacokinetics and safety and local tolerability, respectively.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The trial enrolled 46 patients with both onychomycosis (fungal nail infection) and concomitant tinea pedis (athletes foot).
It evaluated the efficacy signals for tinea pedis at day 42 and for onychomycosis at week 52.
Efficacy evaluations demonstrated significant anti-fungal activity against dermatophytes, the causative pathogens for both onychomycosis and tinea pedis.
The vehicle and active-controlled trial also achieved clinical improvements in both onychomycosis and concomitant tinea pedis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe safety profile of BB2603 was also reported to be excellent, with good local tolerability and no systemic exposure.
Blueberry Therapeutics CEO John Ridden said: “We are delighted that our Phase I/II trial of BB2603 has met both its primary and secondary endpoints and that we are able to use our nanotechnology platform to develop medicines such as BB2603 for diseases where there is a significant medical need, particularly where improved drug delivery or optimised treatment regimes can overcome tolerability and safety concerns and better suit the patient needs.”
Based on the latest results, Blueberry aims to start a Phase IIb dose-finding efficacy study of BB2603 to treat onychomycosis.
Blueberry is expected to apply for an Investigational New Drug Application (IND) with the US Food and Drug Administration (FDA) for BB2603 next year.





