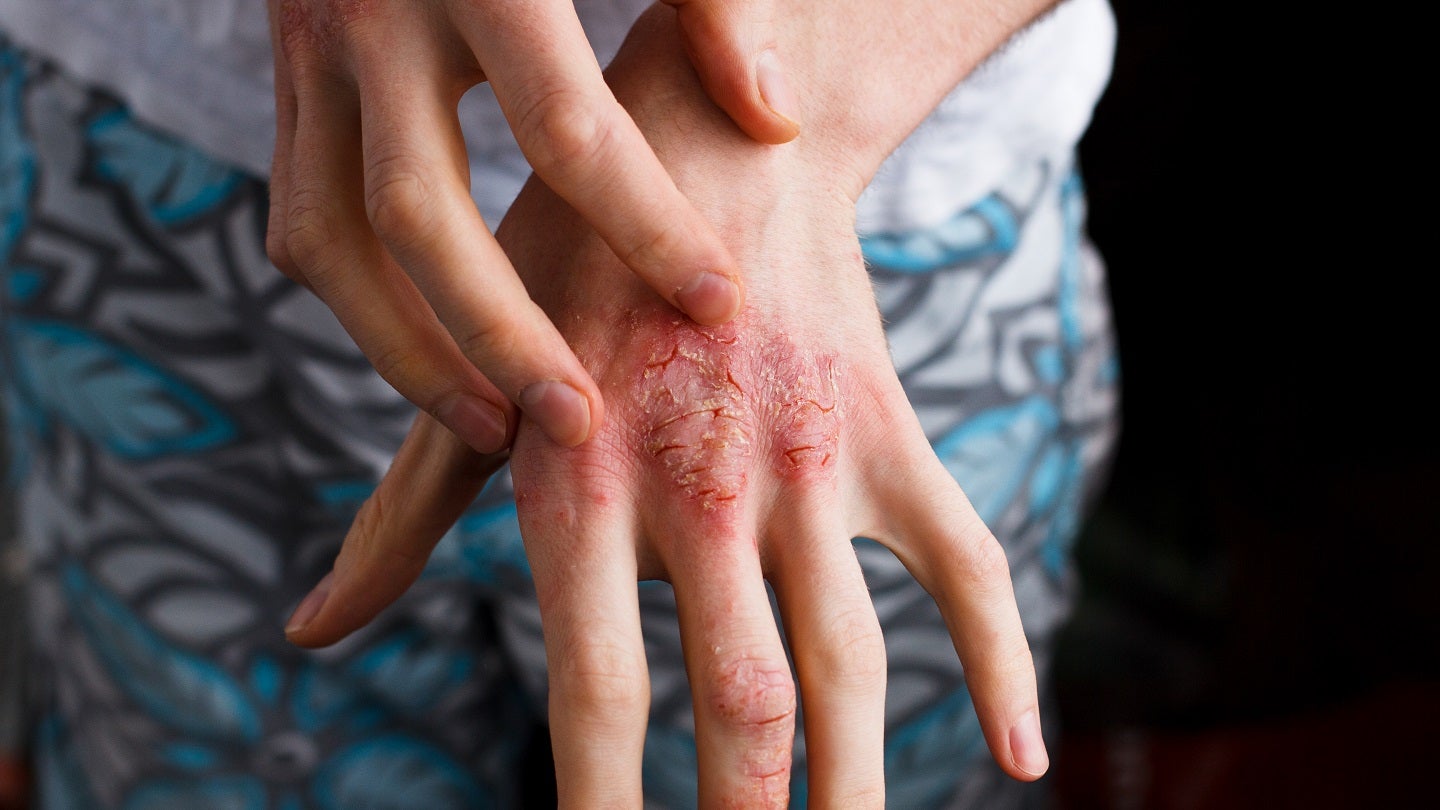
Can-Fite BioPharma has submitted a paediatric study plan to the US Food and Drug Administration (FDA) for treating adolescents with psoriasis using its anti-inflammatory drug Piclidenoson.
Due to Piclidenoson’s favourable safety profile, FDA allowed Can-Fite to enrol adolescents with psoriasis and include them in its two Phase III pivotal clinical studies.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The inclusion of adolescent patients in one or both Phase III studies is believed to significantly broaden any future market launch potential of Piclidenoson.
The data from these trials will be further used by Can-Fite to register the drug with both the FDA and the European Medicines Agency to treat plaque psoriasis.
Can-Fite BioPharma CEO and CFO Motti Farbstein said: “Based on the FDA’s recommendation to enrol adolescents into the upcoming Phase III psoriasis trials, we believe Piclidenoson’s oral formulation with an excellent safety record, combined with its progressive effectiveness over time, make it ideally suited for the chronic treatment of psoriasis in adults and adolescents alike.”
Earlier, favourable results of Piclidenoson were observed in the Phase III placebo- and active-controlled, double-blind, multicentre, randomised COMFORT study for psoriasis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe study met its primary endpoint with statistically significant improvement compared with placebo, as measured by the Psoriasis Area and Severity Index (PASI) 75 response.
Can-Fite’s cancer and liver drug Namodenoson is also being assessed in a Phase IIb trial to treat non-alcoholic steatohepatitis.
The company is also planning to initiate a Phase III trial for hepatocellular carcinoma and a Phase IIa study in pancreatic cancer.





