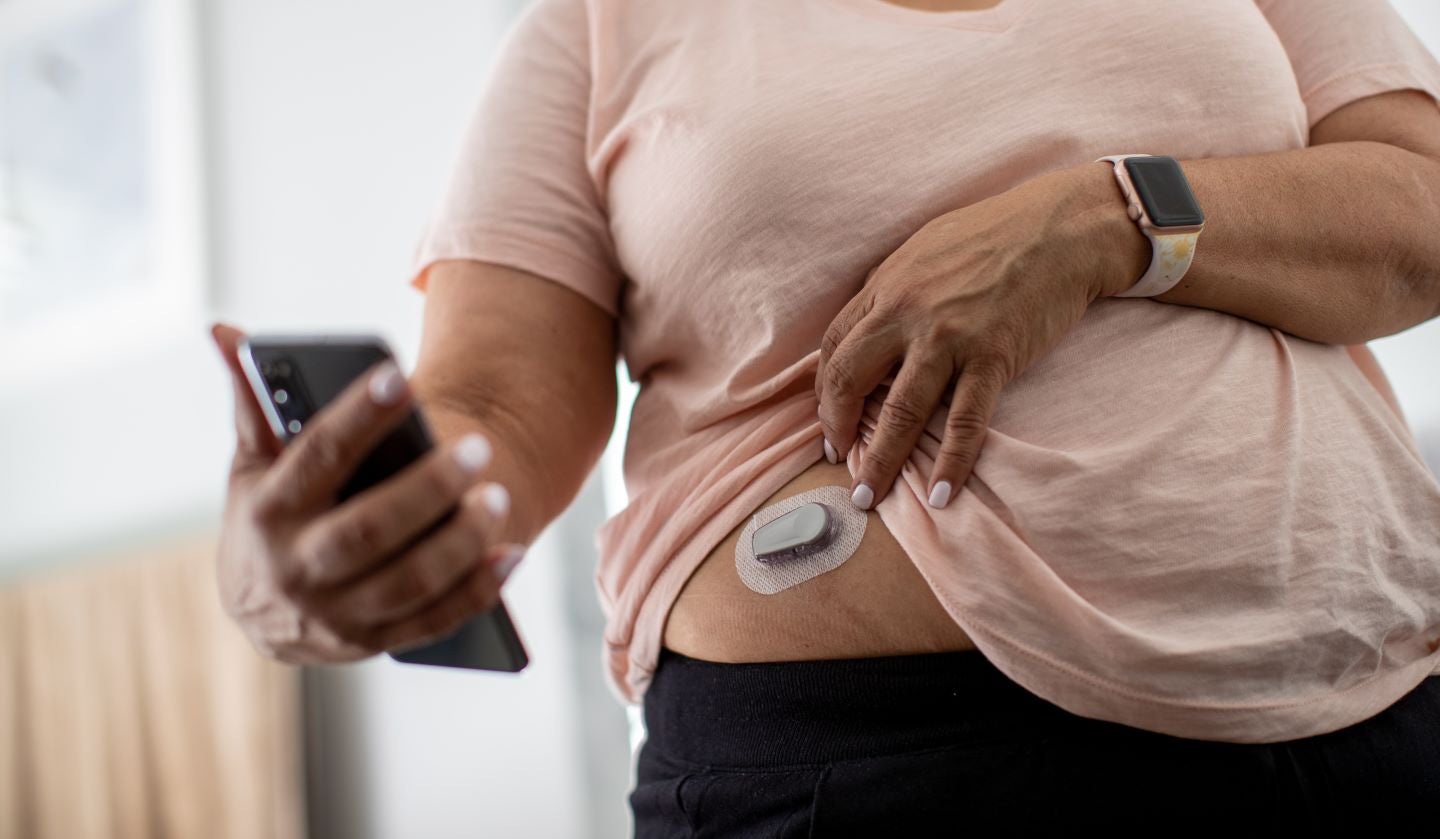
Carmot Therapeutics has begun a Phase II clinical trial of CT-868 in adult subjects who are overweight or have obesity with type 1 diabetes (T1D).
The trial will analyse the impact of CT-868 versus placebo on the per cent variation in HbA1c to week 16 from baseline following treatment.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Various other continuous glucose monitoring (CGM)-associated metrics and appropriate endpoints will also be evaluated in the trial.
Carmot plans to enrol nearly 95 subjects at various trial sites in the US.
Throughout the trial, all subjects will continue to receive insulin therapy with the help of either a pump or several injections on a daily basis and wear a CGM device.
A GLP-1/GIP receptor agonist, CT-868 is administered as a subcutaneous injection once a day. It is in the developmental stage for use as an adjunct to insulin for T1D patients with obesity or who are overweight.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataCarmot chief scientific and medical officer Manu Chakravarthy said: “This Phase II clinical trial represents an important next step in the CT-868 clinical programme—with it, we look forward to evaluating the potential for CT-868 as an adjunctive treatment for people living with T1D.
“We remain very pleased with the progress across all three of our clinical programmes and expect to have multiple data readouts from our obesity and diabetes pipeline throughout 2024.”
The company is also progressing a Phase Ib active comparator crossover trial of CT-868 to assess its impact on glucose homeostasis versus liraglutide in T1D patients.





