A central institutional review board (IRB) has granted study level approval for Skye Bioscience’s Phase II clinical trial of SBI-100 Ophthalmic Emulsion (OE).
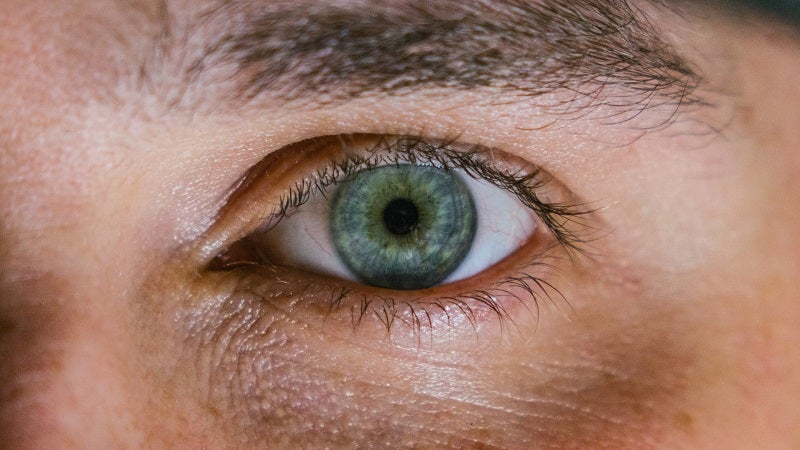
The Phase II clinical trial has been designed for assessing SBI-100 OE in primary open angle glaucoma or ocular hypertension (OHT) patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The prodrug of tetrahydrocannabinol (THC), SBI-100 OE is a new, synthetically derived molecule that is formulated as an eye-drop using a nanoemulsion for improving the delivery of medicine into the eye.
It works by targeting the CB1 receptor that plays an important role in managing intraocular pressure, which is associated with glaucoma.
In animal studies, SBI-100 OE as a monotherapy, as well as in combination with standard of care (SOC) glaucoma drugs, demonstrated favourable results compared to SOC alone and other combinations.
Skye Bioscience CEO and chair Punit Dhillon said: “Following the FDA’s authorisation of our Investigational New Drug application in December, we are methodically completing the manufacturing steps and clinical planning to initiate this important Phase II study of SBI-100 Ophthalmic Emulsion in glaucoma patients in the first half of 2023.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The central IRB approval is another significant clinical milestone toward our Phase II initiation in the US, as we also continue enrolment in our on-going Phase I study in Australia.”
In December, the company dosed the first cohort of healthy participants in its first-in-human Phase I clinical trial in Australia.
Skye Bioscience retained CMAX Clinical Research to facilitate the enrolment of subjects and drug administration to healthy participants in the Phase I clinical trial of SBI-100 OE in April last year.





