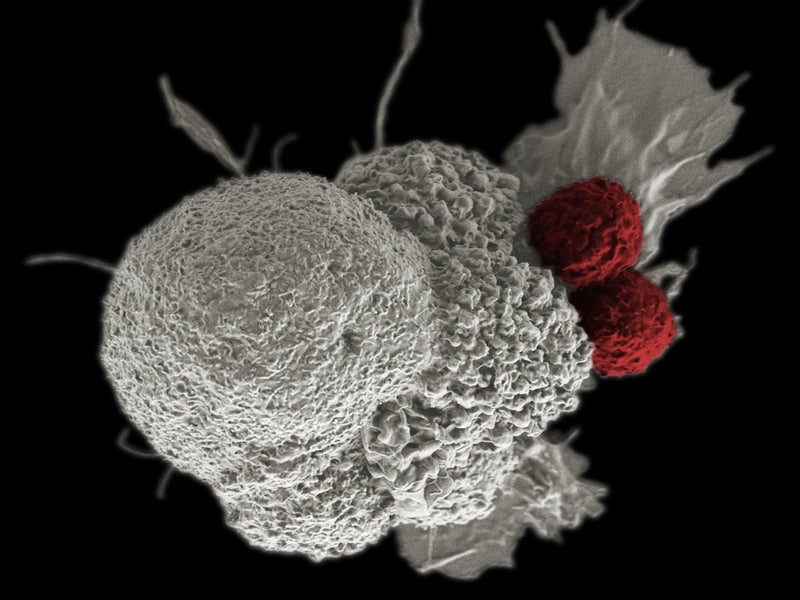
Children’s National Health System in the US has initiated the PLAT-05 clinical trial of a chimeric antigen receptor (CAR) T-cell immunotherapy to treat acute lymphoblastic leukaemia (ALL).
The paediatric hospital is the second site conducting the PLAT-05 trial, following its initial launch at Seattle Children’s.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Designed to simultaneously target the CD19 and CD22 proteins expressed in leukaemia cells, the trial aims to minimise the rate of relapse after CAR T therapy by nearly 50%.
PLAT-05 builds on the findings from the Seattle Children’s Research Institute’s PLAT-02 CAR T-cell therapy trial that targets CD19 protein on cancer cells.
Data showed that following CAR T-cell therapy, leukaemia recurred in certain patients. However, the cancer cells did not have the CD19 protein but manifested the CD22 protein, which the CAR T-cells were unable to recognise and destroy.
As part of the latest trial, researchers at Children’s National will reprogram CAR T-cells to identify and destroy the cancerous cells by targeting both the CD19 and CD22 proteins upfront.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt is expected that the CAR T-cells would be able to still attack the cancer by detecting the CD22 protein even if the tumour evolves to no longer express CD19.
Children’s Research Institute Center for Cancer and Immunology Research director Catherine Bollard said: “We’re excited to bring CD19 and CD22 CAR T-cell therapy to Children’s National for the treatment of paediatric patients with relapsed / refractory acute lymphoblastic leukaemia.
“We hope the PLAT-05 trial leads to increased remission rates for our patients in our effort to make them cancer-free for life.”
The hospital intends to use its participation in the Consortium for Pediatric Cellular Immunotherapy grant to implement the trial, which will enrol patients aged one to 26 with relapsed or refractory pre-B ALL, or other CD19 or CD22-positive acute leukaemia or CD19 non-Hodgkin lymphoma.





