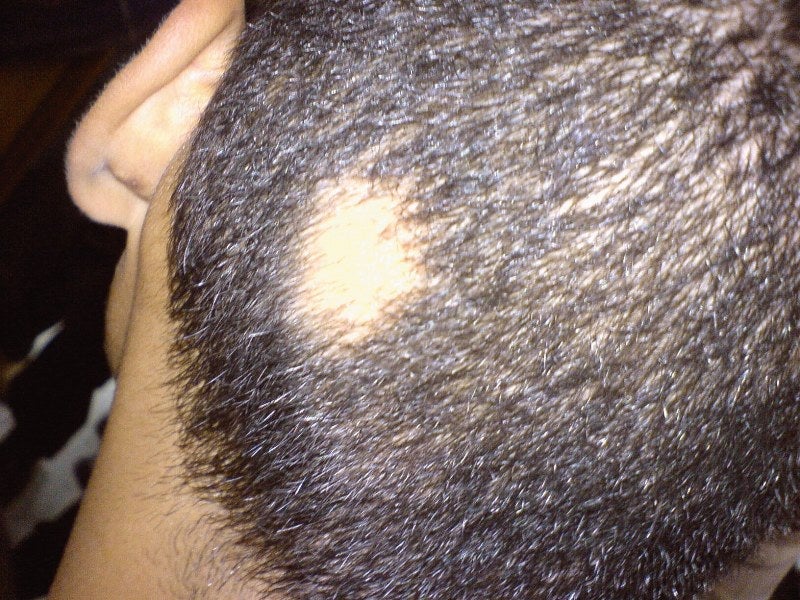
Concert Pharmaceuticals has completed the enrolment of the final cohort of patients in its Phase IIa trial evaluating CTP-543 for the treatment of adults with moderate-to-severe alopecia areata.
Alopecia areata is an autoimmune disease that leads to partial or complete loss of hair on the scalp and body.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Concert is expected to investigate twice daily dose of 12mg CTP-543 in the final cohort.
The company previously studied a 4mg and 8mg twice daily dose of CTP-543 in comparison with placebo.
Data from the trial is expected to be unveiled in the third quarter of this year.
Concert Pharmaceuticals chief development officer James Cassella said: “It is important early on in our development programme to identify the safest and most effective doses of CTP-543 for patients with a chronic autoimmune disease like alopecia areata.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The evaluation of the twice-daily 12mg dose provides a comprehensive exploration of the relevant dose range for CTP-543 and positions us well for later stage development of the compound.”
The Phase IIa trial features a double-blind, placebo-controlled, sequential dose design and is intended to assess the safety and efficacy of CTP-543 among alopecia areata patients.
Patients have been sequentially randomised to be treated with one of three doses of CTP-543 or placebo twice daily.
The trial’s primary outcome will be measured using the severity of alopecia tool (SALT) after 24 weeks of dosing.
Concert revealed positive interim topline results from the 4mg and 8mg twice daily cohorts in November last year.





