CymaBay Therapeutics has announced the termination of Phase IIb and Phase II clinical trials of seladelpar in non-alcoholic steatohepatitis (NASH) and primary sclerosing cholangitis (PSC), respectively.
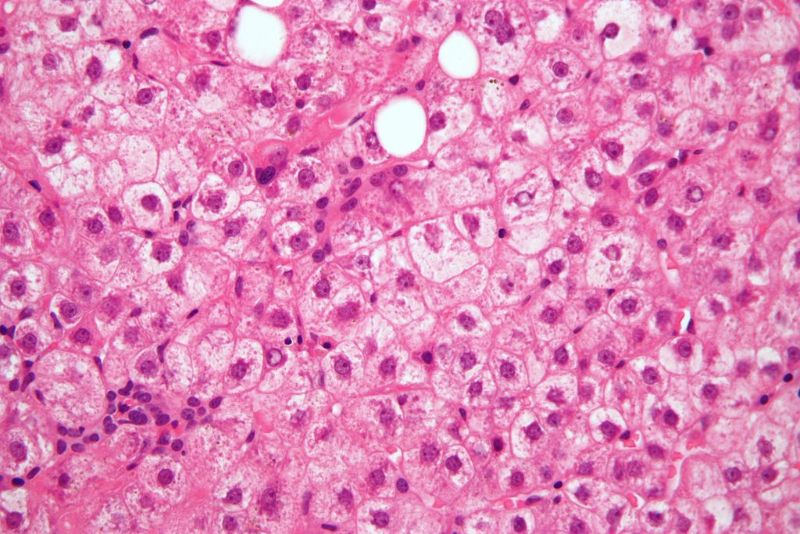
The move comes after biopsies found liver damage in some NASH patients in the Phase IIb study.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Based on the findings, the company also placed all trials of the drug in primary biliary cholangitis (PBC) on hold.
Seladelpar is an oral, selective agonist of peroxisome proliferator-activated receptor δ (PPARδ) being developed to treat inflammatory liver diseases.
The randomised, placebo-controlled, parallel, dose-ranging Phase IIb trial was designed to assess the drug in around 175 NASH patients in the US.
Primary efficacy measure was the change in liver fat content from baseline at 12 weeks.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe Phase II trial for PSC was intended to assess seladelpar in around 100 patients at 60 sites worldwide. Primary efficacy outcome was the change in alkaline phosphatase (AP) from baseline at 24 weeks.
Histological evaluations of the initial set of liver biopsies from the NASH trial revealed ‘atypical’ findings, including an interface hepatitis presentation.
CymaBay added that the findings were observed in participants who showed improvement or stabilisation in biochemical inflammation and liver injury measures.
In addition, these patients did not experience any liver-related adverse events following 52 weeks of treatment.
CymaBay Therapeutics CEO Sujal Shah said: “These findings were also unexpected based on our preclinical and clinical experience with seladelpar to date.
“However, in light of the findings, we have decided to terminate our NASH and PSC studies and place our PBC studies on hold pending further review and follow-up.”
The company has launched an investigation to gain a better insight into the histological findings.





