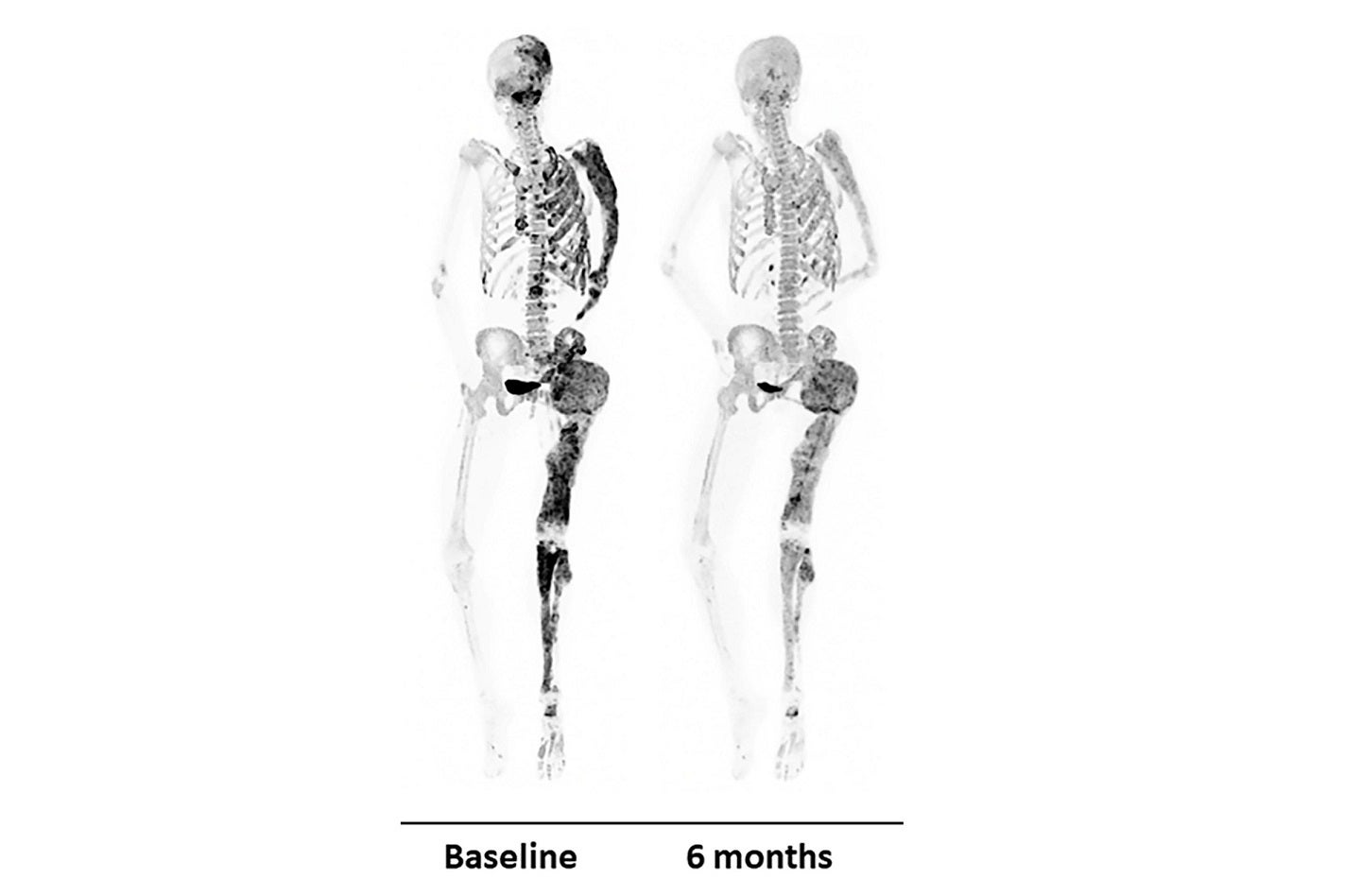
The US National Institutes of Health (NIH) has reported that denosumab significantly reduced abnormal bone turnover in fibrous dysplasia adult patients in a clinical trial.
Bone turnover is a process where old bone is replaced with new bone continuously. It is unusually accelerated in fibrous dysplasia and contributes to bone abnormalities.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The Phase II clinical trial was conducted by researchers from the NIH Clinical Center and the National Institute of Dental and Craniofacial Research (NIDCR) in eight women, who received high denosumab doses for six months.
Published in the New England Journal of Medicine, the findings demonstrated that denosumab might improve quality of life of patients by enabling healthy formation of bone.
The scans and bone biopsies of the participants demonstrated reduction in bone turnover in lesions, at the end of the treatment period.
According to the findings, proteins in the blood that are associated with bone turnover dropped to normal levels, and patients had reduction in complications while receiving treatment with denosumab.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataNIDCR clinical investigator Alison Boyce said: “Surgery is still the standard treatment for fractures and deformities caused by fibrous dysplasia.
“Denosumab is the first medication that appears to affect how fibrous dysplasia lesions behave and improves patients’ disease outcomes.”
The US Food and Drug Administration licensed denosumab for the treatment of bone problems caused from osteoporosis and cancer.
The medication works by blocking receptor activator of nuclear factor kappa-Β ligand (RANKL) protein that is elevated in fibrous dysplasia patients.





