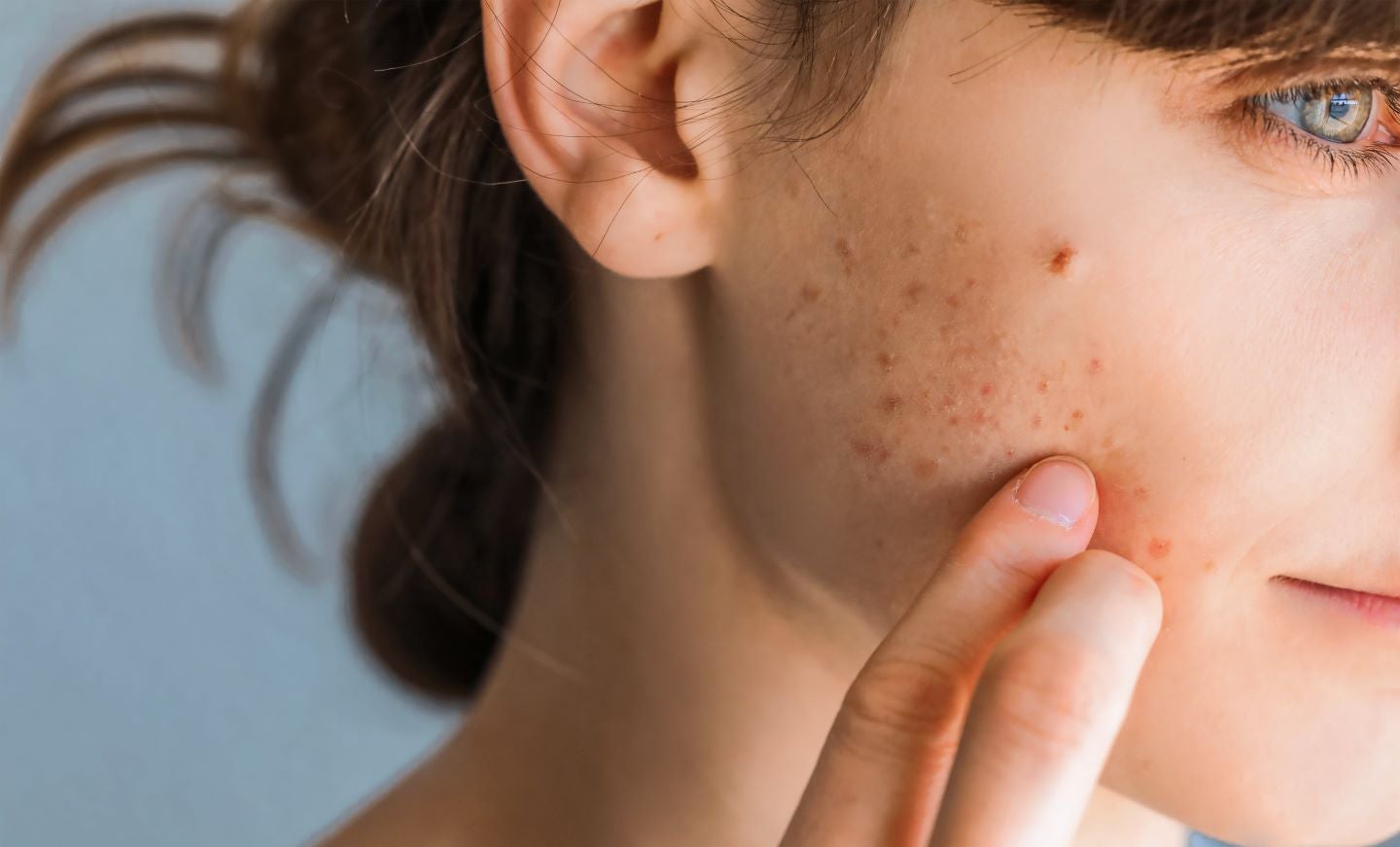
Dermata Therapeutics has enrolled the first subject in the Phase III STAR-1 clinical trial of its topical product candidate, DMT310, to treat moderate-to-severe acne.
STAR-1 is the first of two Phase III studies in the DMT310 Phase III clinical programme to assess the safety, efficacy and tolerability of the product for patients with facial acne.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This double-blind, and placebo-controlled trial will randomise subjects aged nine years and above in the US and Latin America into a 2:1 ratio to receive either DMT310 or placebo.
The mean variation in inflammatory and noninflammatory lesion counts from baseline and the Investigator Global Assessment (IGA) treatment response rate is the trial’s primary endpoint.
Dermata anticipates reporting topline data from the STAR-1 trial in the first quarter of 2025.
Obtained from freshwater sponges, DMT310 is in development to treat various skin ailments.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn a prior Phase IIb trial, once-a-week use of the product offered statistically significant results for all primary and secondary endpoints to treat moderate-to-severe acne at all time points.
Nearly 45% of the trial subjects in the DMT310 arm attained an IGA score of clear or almost clear versus below 18% in the placebo arm by 12 weeks.
Dermata chief development officer Christopher Nardo said: “Getting the first patient enrolled in this Phase III programme is an important step in the potential approval of DMT310 as a first-in-class treatment for the millions of patients suffering from acne.
“DMT310 has demonstrated significant improvement for both inflammatory and noninflammatory facial lesions in our Phase IIb study, and we are excited to attempt to confirm this efficacy in the pivotal Phase III clinical programme.
“We look forward to providing additional updates on the progress of this programme and anticipate topline results from the STAR-1 study in early 2025.”
Apart from acne indication, DMT310 was analysed to treat psoriasis and rosacea.





