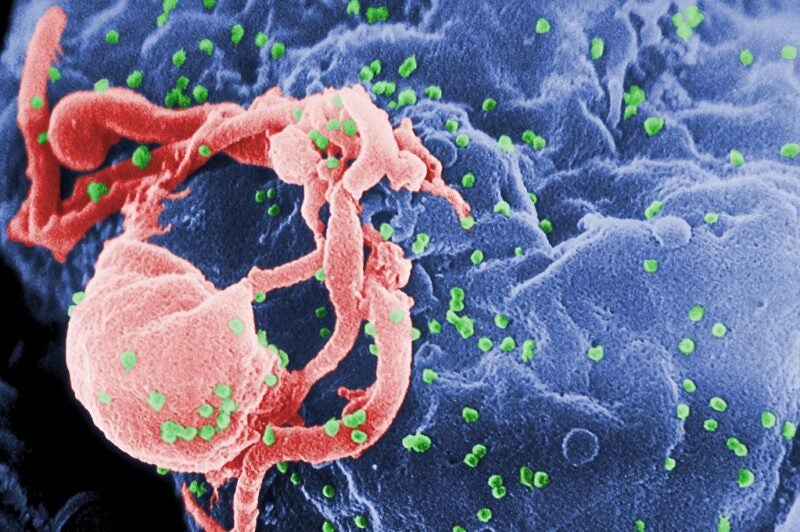
Excision BioTherapeutics has dosed the first subject in the Phase I/II clinical trial of its CRISPR-based therapeutic EBT-101 to treat human immunodeficiency virus type 1 (HIV-1).
The multicentre, first-in-human, open-label, single ascending dose trial will assess the tolerability, safety, and initial efficacy of EBT-101 in nearly nine HIV-1 patients who are suppressed on antiretroviral therapy (ART).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Evaluating the safety and tolerability of one dose of EBT-101 in subjects with an undetectable viral load on ART is the trial’s primary objective.
The trial will also carry out pharmacodynamic, biodistribution, and efficacy analyses.
At week 12, all subjects will be evaluated for eligibility for an analytical treatment interruption (ATI) of the ART.
The trial is based on preclinical data that comprised positive, durable, non-human primate safety data and efficacy data from transgenic mice.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe findings showed that EBT-101 treatment could potentially cure HIV.
EBT-101 is created for curing HIV infections using a single intravenous infusion.
It is said to eliminate HIV proviral DNA from impacted cell reservoirs.
For delivering CRISPR-Cas9 and dual guide RNAs, EBT-101 uses an adeno-associated virus (AAV). This allows for a multiplex editing approach to act on three separate sites within the HIV genome at the same time.
In turn, this permits the excision of the HIV genome’s large portions to reduce potential viral escape.
Excision BioTherapeutics CEO Daniel Dornbusch said: “It is the first time a CRISPR-based therapy targeting an infectious disease has been administered to a patient and is expected to enable the first ever clinical assessment of a multiplexed, in vivo gene editing approach.
“With this achievement, Excision has taken a major step forward in developing a one-time treatment that could transform the HIV pandemic by freeing affected people from life-long disease management and the stigma of disease.”





