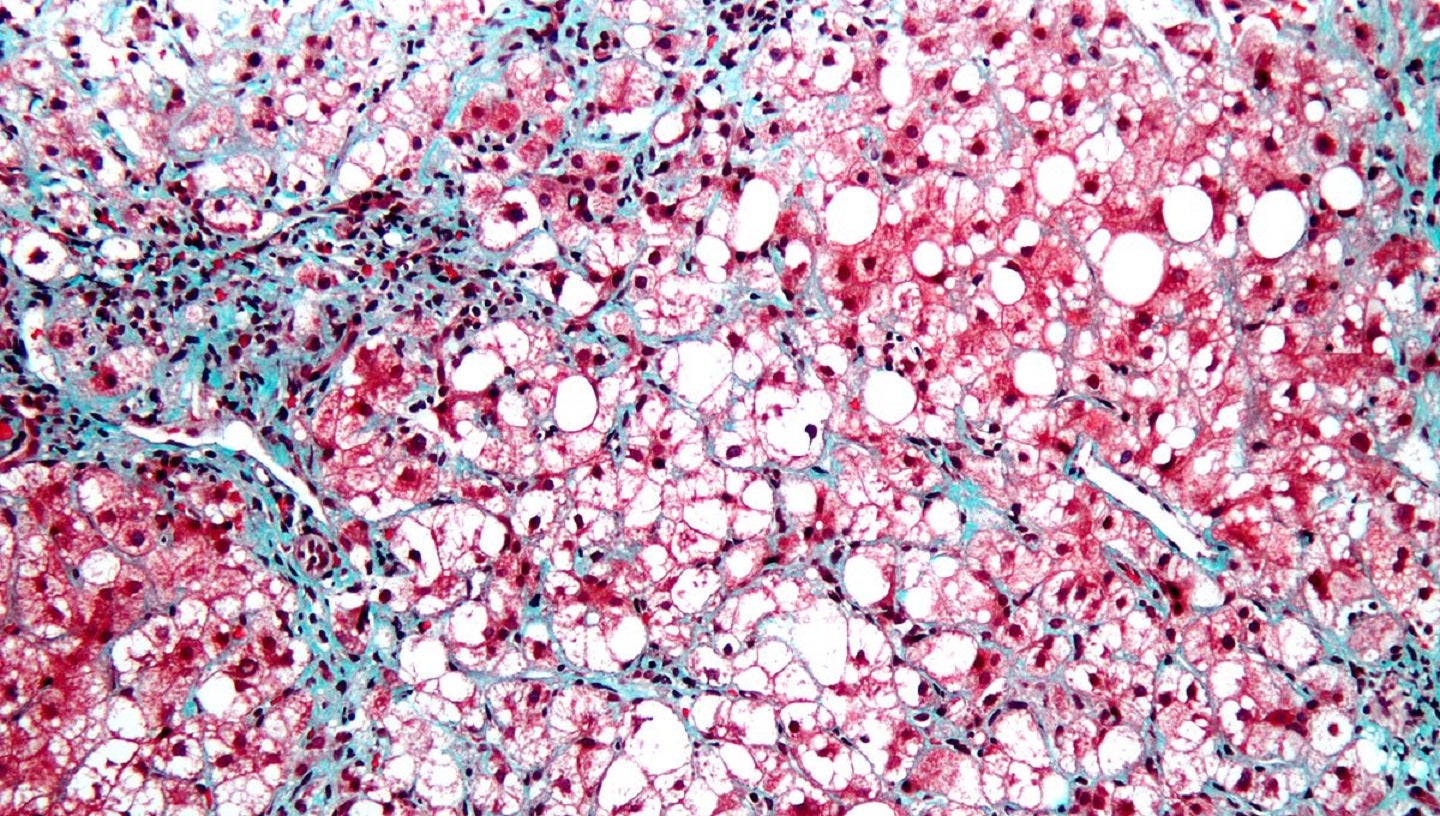
The US Food and Drug Administration (FDA) has granted clearance to NeuroBo Pharmaceuticals’ investigational new drug (IND) application enabling initiation of a two-part Phase IIa clinical trial of DA-1241 to treat nonalcoholic steatohepatitis (NASH).
Designed to evaluate the efficacy and safety of DA-1241, the 16-week, randomised, multicentre, double-blind, placebo-controlled, parallel clinical study will enrol subjects with presumed NASH and confirmed pre-diabetes or T2DM.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Part I of the study will evaluate the efficacy of DA-1241 against a placebo while Part II in combination with sitagliptin versus placebo.
Part I and Part II are expected to enrol around 49 subjects and 37 subjects, respectively.
Three treatment groups of Part I will receive 50mg, 100mg of DA-1241 and placebo in a 1:2:1 ratio while two treatment groups of part II will be randomised into 2:1 ratio to receive DA-1241 100 mg/sitagliptin 100mg or placebo.
Change from baseline in alanine transaminase (ALT) levels at week 16 is the primary endpoint for both the studies.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe proportion of participants with normalisation of ALT, absolute change in liver fat from baseline, relative percent change in liver fat fraction from baseline, and proportion of subjects with a reduction of 30% or more in liver fat from baseline, among others are considered as secondary efficacy endpoints.
NeuroBo interim president and CEO Joe Hooker said: “We are eager to initiate the two-part, Phase IIa clinical trial of DA-1241, which we expect will occur in the third quarter of this year.
“The two-part design provides optionality for an interim analysis in the first half of 2024 and we anticipate full data in the second half of 2024.
“Looking ahead, we expect additional, near-term value creating milestones, including advancement of DA-1726, our second asset, through the IND process, with the goal of initiating a Phase Ia safety study in the first half of 2024 and an expected data readout in the second half of 2024.”





