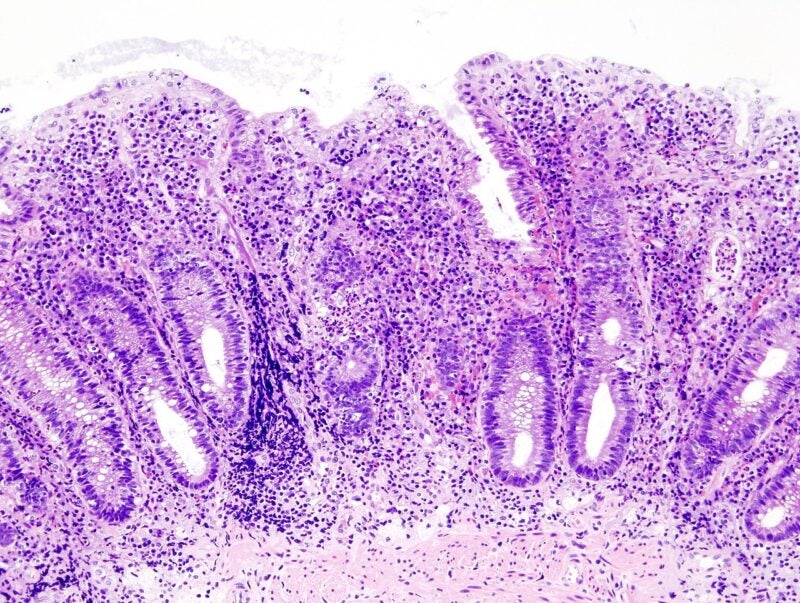
The US Food and Drug Administration (FDA) has granted clearance for Asieris Pharmaceuticals’ Investigational New Drug (IND) application to begin a Phase Ib clinical trial of its oral drug, APL-1401, to treat moderately-to-severely active ulcerative colitis (UC).
Asieris intends to subsequently commence subject enrolment in the trial in the country.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The randomised, double-blind trial will assess the safety, tolerability, pharmacokinetics, and initial efficacy of APL-1401 in such patients.
Asieris Pharmaceuticals chief operation officer Dr John Zhuang said: “As the world’s first oral drug that modulates neurotransmitters to suppress inflammation independently developed by Asieris, APL-1401 will hopefully provide a new treatment for UC patients.
“APL-1401 was born from Asieris’s technology platform for immune regulation.
“We expect to get safety data and preliminary efficacy signal in this clinical study as soon as possible to support the subsequent clinical development of the product and clinical studies for other indications.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe company also plans to file a CTA application with the National Medical Products Administration (NMPA) of China for the drug soon.
A chronic idiopathic inflammatory bowel disease (IBD) of the colon, UC leads to superficial mucosal inflammation from the rectum to the more proximal colon.
At present, there exists no treatment for UC.
In June 2019, the company concluded subject enrolment in its Phase Ib clinical trial to assess the safety, tolerability, and PK characteristics of APL-1202 in the US.
APL-1202 is an oral and reversible methionine aminopeptidase II type (MetAP2) inhibitor.
The trial analysed APL-1202 along with intravesical BCG in NMIBC patients.





