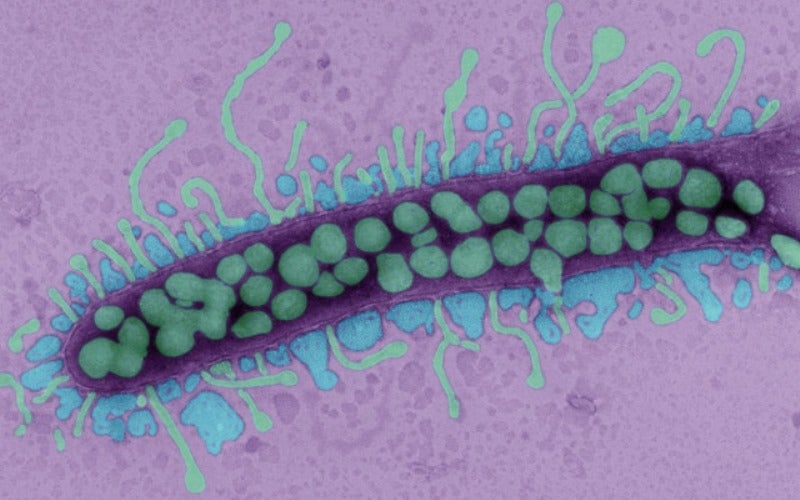
A team of physician-scientists at University of California San Diego School of Medicine (UC San Diego) has received approval from the US FDA to conduct a trial of AB-SA01 bacteriophage therapy for the treatment of ventricular assist devices (VADs) infected by Staphylococcus aureus (S. aureus).
Approval was granted to an investigational new drug application submitted by the team.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The proposed Phase I/II trial would see the team of physicians investigate the safety, tolerability, and efficacy of intravenous administration of AB-SA01 in combination with existing antibiotic therapies.
It is expected to enrol around ten subjects at UC San Diego Health and other teaching hospitals in the US.
Bacteriophages specifically target and consume bacteria. The ubiquitous virus is present at any place where bacteria exist and was once considered to be a promising therapy.
The trial’s principal investigator Saima Aslam said: “There is a high unmet need in patients with S. aureus VAD infections, which are typically very difficult to eradicate with conventional antibiotic therapy.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“In 2018, our UC San Diego Health team treated a patient with a S. aureus VAD infection using AB-SA01 under AmpliPhi’s Expanded Access Programme.
“This clinical trial builds on that foundational work and could provide a much-needed and promising treatment option for this life-threatening condition.”
UC San Diego has formed a partnership with US-based biotechnology company AmpliPhi Biosciences for the trial.





